International Journal of
eISSN: 2475-5559


Research Article Volume 4 Issue 2
1Department of Mechanical Engineering, Copperbelt University, Zambia
2Department of Energy Engineering, Budapest University of Technology and Economics, Hungary
Correspondence: Lennox Siwale, Department of Mechanical Engineering, Copperbelt University P.O Box 21692, Kitwe Zambia, Tel l+27764422347, +27 12 382 5164, Fax +27 12 382 5602
Received: May 27, 2018 | Published: April 4, 2019
Citation: Siwale L, Bereczky A, Chama S, et al. Effect of oxygenated fuels on emissions characteristics: a comparative study between compression ignition and spark ignition engines. Int J Petrochem Sci Eng. 2019;4(2):57-64. DOI: 10.15406/ipcse.2019.04.00104
It is agreed by scientists world-wide that continued burning of petroleum oils without intervention is a great threat to the environment. In this study a comparison is made of the extent of emissions produced between diesel and gasoline engines using oxygenated blends. In the gasoline engine 20% methanol -80%, gasoline M20 was used. In the diesel engine, 20% n-butanol and 80% diesel B20 was the test fuel. The gasoline engine was a naturally aspirated Suzuki RS-416 1.6L engine type and the diesel type engine was a 1Z type, 1.9L Turbo-Direct injection (TDI). The results obtained were as follows: the NOx emissions increased with an increasing BMEP for Diesel Fuel (DF) but was slightly lower than the blend B20 at 50 and 75 % load; whereas using M20, Nox reduced in reference to gasoline fuel (GF) but was four times higher than that obtained in diesel engine; using B20 diminished the quality of Unburned hydrocarbons (uHc) emissions in diesel engine based on the reference fuel DF. The range of emissions of uHC however was far less in the diesel engine than in the gasoline engine.10-60 ppm and 600 to 700 ppm respectively. The blend M20 reduces uHc more than the GF above 25% brake mean effective pressure (bmep).The formation of Carbon monoxide (CO) was rapid for M20 than GF; emission concentration of CO in B20 increased above DF. Exhaust gases temperature (EGT) was lower for all oxygenated blends, M20 and B20, than neat or pure hydrocarbon (HC) fuels: GF and DF.
Keywords: n-butanol, methanol, gasoline, emission, compression ignition, spark ignition engine
The goal to replace or reduce the use of petroleum oil because of the environmental degradation that conventional fuels such as gasoline and diesel causes looms on. Use of petroleum oil in transportation greatly contributes to the deterioration of the environment through the emission of regulated emissions such as nitrogen oxides (NOx), unburned hydrocarbon (UHC), carbon monoxide (CO), particulate matter (PM) and carbon dioxide (CO2). The CO2 pollutant accumulating into the atmosphere contributes to the greenhouse gases (GHG) effect, global warming and climate change. Unburned hydrocarbon and soot cause respiratory problems and are carcinogenic. Oxygenated fuels such as alcohols have been used extensively by researchers world-wide to reduce the negative effects on the environment by burning petroleum oil. Several studies have been performed on compression ignition (CI) engines with regards to engine performance and the characteristics of exhaust emissions, where alcohol fuels namely methanol and ethanol are added into diesel fuels.1 It has been reported that during cold starting, exhaust gases in diesel engines make a larger contribution to the total emissions than at any other operating position of the engine. Consequently, cold starting of engines has gained increased attention.2 Methanol has the lowest combustion energy and because of its highly polar nature, methanol does not mix with diesel fuel. Ethanol, on the other hand, can be mixed with diesel provided that the ethanol is anhydrous or has little water in it. The low CN of ethanol impedes its use in diesel fuel. However, butanol can be blended with diesel fuel at any concentration and does not separate when mixed with water or at a low temperature. The CN however, impedes the significant use of butanol, which diminishes the auto-ignition capability in diesel fuel. Hence to overcome problems associated with the use of methanol, ethanol and butanol require further studies to identify suitable additives or CN improvers.3 While anhydrous ethanol is miscible in gasoline, its miscibility with diesel is problematic. However, recent interest in simultaneous use in diesel of biodiesel, ethanol and n-butanol has arisen. However, since biodiesel is characterised by high viscosity, lubricity, CN and flash point relative to that of ethanol, the obstacles due to ethanol are partially overcome. Literature concerning the use of n-butanol/diesel fuel blends in diesel engines and their effect on the steady-state performance and exhaust emission in engines is limited, but with a steady rising trend.4,5 It has been reported that 186 research studies have been published since 2005 on the alcohol-diesel issues.3 Butanol studies include investigations using different types of engines such as: two-cylinder, naturally-aspirated or turbo-charged, light or heavy-duty. Approaches also include blending alcohol with vegetable oils to improve solubility of the vegetable oils to reduce viscosity to aid flow ability.6 Some studies have focused on vegetable oils blended with ethanol and n-butanol to test performance in a diesel engine. In addition, in one study butanol/diesel was used in a light-duty turbo diesel vehicle.7 The n-butanol fuel has higher-energy content (28.4 MJ/L) than ethanol (21.2 MJ/L).7 While it is argued by others that alcohol extends the ignition delay in a diesel engine,8 certain researchers, however, point out that the emissions in the exhaust gases are reduced,7 conducted experiments on a diesel engine. CO emission is similar for all the fuels but higher for DF at low BMEP. The blends that were used contained 8%, 16%, 24% n-butanol with diesel. The blends indicate higher UHC emissions for the same BMEP than that of DF. The emissions of UHC also increase with BMEP for the test fuels but decrease as the alcohol fraction in the blend is decreased. Carbon monoxide was lowest at medium BMEP for the bus diesel engine9 for all the fuels. The carbon monoxide concentration was lower for B8 than B16, which was almost the same as for B0. All the blends show higher UHC emission than that of DF. Similar results of regulated emission are obtained on a two-stroke diesel engine10 and a direct-injection, (DI) single-cylinder diesel engine7,8 also conducted experiments on a single-cylinder, naturally-aspirated, direct-injection (DI) standard Ricardo-Cusson diesel engine. They found that the NOx emissions increase with an increasing BMEP for DF that is higher than the blends B8, B16 and B24. The reasons for these differences are explained in their paper. They believe the differences were caused by the lower calorific value, the higher heat of vapourisation, and the lower CN of oxygenated butanol. NOx increases for the test fuels but is less for the blends than for DF at a low speed. In a review by,11 the engine load was reported to play an important role in the formation of NOx. More fuel or a richer mixture is needed to increase the engine load, which results in a higher in-cylinder temperature and NOx. Increasing engine speed was reported to have similar effects on NOx as with the load. In a study carried out by9 on engine performance and emission, the characteristics were investigated for butanol blends with DF on a bus diesel engine. The blend fuels were B8 and B16, the reference fuel was (B0). In all cases, the increase of smoke opacity with speed was observed. Smoke opacity increases with BMEP. The highest values were obtained for DF. This is in agreement with the results obtained in their earlier investigations.7 BSFC slightly decreases with increasing load for all the fuels but is higher for the blends than for DF.9 The blends have a lower heating value than diesel. This causes the engine to demand more blended fuel to be delivered than when fueled with diesel only in order to maintain the same engine-brake power output. BSFC is observed to increase with retarded main injection timing. The cited authors tested blends of 8% and 16% n-butanol/diesel on a bus diesel engine. In a triple injection strategy,12 used a heavy-duty, direct-injection diesel engine on pilot-main-post injection. In their research, the BSFC increased when the main injection timing was retarded. This occurs because the brake power drops. B5, B10, and B15 were used. Some studies on a 4.7kW diesel engine were carried out to evaluate the performance, combustion and emission characteristics of a blend of used palm oil (UPO), a vegetable oil in diesel. With the increased amount of UPO in diesel, the ignition delay reduces because of an earlier ignition of volatile compounds associated with UPO during injection. Ignition delay decreases as the load is increased probably due to high in-cylinder temperatures at high loads causing easy evaporation of the injected fuel. Adding n-butanol to the UPO-diesel blend increases the ignition delay. This occurs because of the reduction in the CN and increase of the latent heat of vaporisation of the blend caused by the addition of n-butanol.13 A study conducted on a direct-injection diesel engine demonstrates that the average increase of BSFC above that of DF is between 2.06% and 8.55% with iso-butanol ISB15 and ISB20 respectively.14 conducted7 experiments on the Ricardo-Cusson diesel engine. The EGT is higher at high BMEP for all the fuels. DF has the highest EGT for the given operating range of BMEP but only slightly higher than that of the blends. The B8, B16, and B24 blends were used. The reasons for these differences have to do with the higher heat of vapourization of the alcohol blends.7 The BTE is the same for all the fuels and increases with increasing BMEP in the study. The blends used were B8 and B16.9 The BTE is relatively high when a blend of up to 10% isobutanol content is used in DF in a direct-injection diesel engine in a study conducted by.14 This is attributed to the oxygen content of the blends. Besides, blends with up to 10% butanol content, do not show significant changes or decrease of energy-content and CN of the fuel. The BTE slightly improved at high speed for blends containing up to 10% isobutanol. The author reports that this is attributed to compensation of a lower CN of iso-butanol blends via high cylinder temperatures, flame-speed propagation, with improved vaporisation and mixing process of the fuel and air leading to a shortened ignition delay. A noticeable decrease in BTE with ISB15 and ISB20 is observed. These results can be attributed to a combined effect of considerable reduction of brake power and increase in fuel consumption.14,15 conducted experiments on a port fuel injection spark ignition engine. The researchers found that specific UHC, CO and NOx emissions fueled with gasoline and n-butanol blends are lower than those of GF. They observed that pure n-butanol increases the specific UHC and CO emissions while the specific NOx emissions decrease compared to those of GF. They reported that less than 40% n-butanol in the blend with GF can reduce UHC emission at different loads compared to those of GF. Pure n-butanol, on the other hand, yields higher UHC and CO compared to that of GF due to the drop in temperature of the gas as n-butanol evaporates, thus decreasing the UHC and CO oxidation during the expansion and exhaust processes. They explained that retarding the spark timing would increase the in-cylinder temperature in the expansion and exhaust processes and facilitate the UHC to be converted to CO and CO to CO2. They reported that advancing the spark timing, on the other hand, increases the engine specific UHC, NOx emissions and decreases CO emissions. They stated that early combustion would increase cylinder peak pressure as well as maximum temperature causing the NOx emission to increase. However, the temperature in the expansion and exhaust processes decreases, which slows down the oxidation of UHC. They observed that pure n-butanol yielded the lowest specific NOx due to the low adiabatic temperature and heating value and at the same time increases CO. it is observed from the literature that studies were often done to compare conventional fuels with their blends of oxygenated greener fuels. However, across the engine type’s: compression and spark ignition engines (CI and SIE’s); comparisons of the same blends or similar percentage of total alcohol are not widely reported. In this study the effects of oxygenated fuels across the two engine type mostly used in transport sector: CI and SIE’s are compared in terms of emissions of uHC, NOx and CO.
The objectives of the study were twofold as follows:
The n-butanol alcohol was manufactured by VWR Prolabo (BDH), with 99.99 % purity, and density of boiling point: 118oC (at 101.3kPa), melting point: -89.8oC, and flash point: 30oC. The methanol had purity of 99.9%, and was manufactured by Molar Chemicals (KFT 1151 Budapest). It has density of 790 kg/m3 (20oC), water content of 0.028 %, and evaporation residual: 0.0008 %. The characteristics of diesel used for the experiments were: D2, standard EN 590, CN, ≥51, sulphur content≤10mg/kg; water content≤200mg/kg; and kinematic viscosity 2.00 to 4.5 (mm2/s) at 40o C, specific density at 15 oC, ≥0.82; and flash point of >55oC. Gasoline was manufactured by MOL with a specification of EN-228 of octane number 95.
The test facility for the four-cylinder NA and SI engine
The engine used was Suzuki (66kW) RS-416, four-cylinder, spark ignition engine, with sixteen valves; in-line; four-stroke; multi-point injection (Figure 1). The overview of the engine test bed, air intake, exhaust lines, fuel injection, and measuring devices for emissions and data collection are illustrated in Figure 2. The technical specification and engine parameters are listed in Table 1.
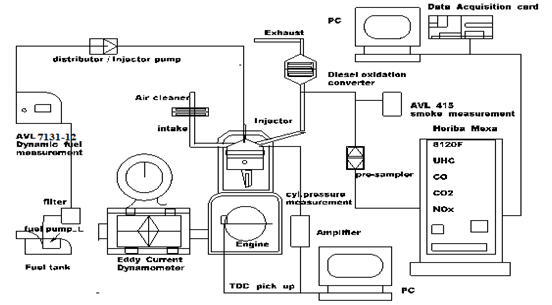
Figure 2 Layout of the diesel engine test bed, emission measuring equipment, data display and collection equipment.
Engine type |
Suzuki Rs-4161.6L |
Model |
T10M16A (2006) |
Bore [mm] |
78 |
Stroke [mm] |
83 |
Swept volume [l] |
1.586 |
Compression ratio [-] |
11.1 |
Power (kW) |
92 (@6800 RPM) |
Max torque (Nm) |
148 (@4800 RPM) |
Number of cylinders |
4 |
Camshaft |
DOHC with VVT |
Number of valves |
4 |
Fuel type |
Petrol-P |
Fuel aspiration |
Naturally aspirated |
Fuel delivery |
Multi-Point Injection |
Table 1 Technical specification for the Suzuki (66kW) engine16
The test facility for the four -cylinder CI engine
Figure 2 shows the layout of the diesel engine test bed, instrumentation, air intake, fuel delivery, emission measuring devices and data display unit. The four-cylinder compression ignition engine used was the 1Z type, 1.9L-66kW Turbo-Direct-injection (TDI) Volkswagen diesel engine. The technical specification and engine parameters are listed in Table 2.
Type |
1Z 0159 (1994) |
Bore |
79.5 [mm] |
stroke |
95.5 [mm] |
Swept volume |
1.9 [L] |
Compression ratio |
19.5 [-] |
Maximum Torque |
202[Nm]/1800 |
Maximum power |
66kW/4000 [RPM] |
Fuel system |
|
Injector pump |
Distributor-type (Bosch VP37) |
Combustion chamber |
Bowl in piston |
Injector type |
5 hole ,180 [bars] |
Table 2 Engine parameters 1Z type, 1.9L Turbo-Direct injection (TDI) Volkswagen diesel engine17
Measurement of nitrogen oxides concentration
NOx was measured by means of a chemiluminescence detector (CLD) of the type CLD-53M. The detector allowed the NO/NOX content to be measured in the range 0 and 5000ppm.
Measurement of fuel-mass flow
The fuel-mass flow rate was measured using the AVL-7131-12 dynamic fuel-consumption measuring equipment. The fuel balance works on the gravimetric measuring principle. Fuel is supplied to the engine from a measuring vessel inside the instrument where the weight of the fuel is continuously measured. This instrument enables the highest temperature stability of the fuel conditioning system with a measuring accuracy of 0.12%; including self-calibration according to ISO 9001, the technical detail of the unit is presented in Table 3. The accuracy of the fuel balance depended on the measured mass of the fuel (g), with measuring time of 30sec (Table 3). The test blends are given in Table 4.
Item |
Description |
Specification or type |
Accuracy |
1 |
Eddy current Dynamometer |
Borghi & Saveri FE350s |
Moment of inertia 0.681 kg/m2 |
2 |
Fuel flow meter |
AVL- 7131-12 |
± 20g/h |
3 |
Gas analyser, CO |
Horiba model infra red AIA-23 |
± 2% of Full Scale Deflection (FSD) |
4 |
Gas analyser, UHC |
Horiba model FID;FIA-22 |
±2% of FSD |
5 |
Gas analyser, NOx |
Horiba CLD,CLD-53M |
± 2% of FSD |
9 |
Crank angle trigger/ Octane rating engine |
Kübler 8.5822.3851.1024 |
1024 pulses/round, max 6000 RPM |
Crank angle encoder /TDI& Suzuki engines |
Optical encoder Henstler RI 32-0/1024.ER |
1024 pulses/round, max 6000RPM |
|
10 |
Charge amplifier |
Kistler KIAG 5001 |
- |
11 |
Temperature sensor |
Type K |
± 1.1oC |
Table 3 Accuracy of measuring equipment
Blend # |
Methanol (%) |
n-butanol (%) |
Gasoline (%) |
Ctot (%) |
Identification |
1 |
- |
- |
100 |
0 |
M0 |
2 |
20 |
0 |
80 |
20 |
M20 |
Diesel (%) |
|||||
3 |
- |
- |
100 |
0 |
B0 |
4 |
20 |
80 |
20 |
B20 |
|
Table 4 Gasoline/methanol/n-butanol test fuel and blends
Test fuels properties
The properties of methanol, ethanol, butanol, gasoline and diesel are compared in Table 5 and Table 6.
Methanol |
Ethanol |
Gasoline |
*Butanol |
|
Molecular formula |
CH3OH |
C2H5OH |
- |
C4H9OH |
Oxygen content (%) |
50 |
46 |
0 |
22 |
Density [kg/m3] |
792 |
785 |
740 |
810 |
LHV [MJ/kg] |
20 |
26.9 |
44.3 |
33.3 |
Octane Number |
111 |
108 |
>90 |
113 |
Auto-ignition temp [oC] |
465 |
425 |
228-470 |
385 |
Stoichiometric (A/F) ratio[kg/kg] |
6.47 |
9 |
14.8 |
11.1 |
Latent heat[kJ/kg] |
1103 |
840 |
305 |
581.4 |
Vapour pressure at 23.5oC [kPa] |
3.2 |
- |
60-90 |
32 |
Fuel properties |
Diesel fuel |
n-butanol C4H9OH |
Ethanol C2H5OH |
Density at 20 (oC, kg/m3) |
837 |
810 |
788 |
Cetane number |
50 |
~25 |
~8 |
Lower calorific value, MJ/kg |
43 |
33.1 |
26.8 |
Kinematic viscosity at 20oC, mm2/s |
3.4a |
3.6b |
1.52b |
Boiling point, oC |
180-360 |
118 |
78 |
Latent heat of evaporation, kJ/kg |
250 |
585 |
840 |
Oxygen, %wt. |
0 |
21.6 |
34.8 |
Stoichiometric air-fuel ratio |
15 |
11.2 |
9 |
Molecular weight |
170 |
74 |
46 |
Procedure and engine test variables on naturally-aspirated, Suzuki engine
The engine was warmed up until the cooling water temperature rose from 22˚C to 90˚C. The electronic control unit, (ECU) used was the versatile engine management system VEMS 3.3. Details of the measuring devices are described in Section 2.1. The following dependencies between manipulated (independent) and controlled (dependent) variables (also referred to as effects) were considered in the experimental investigation. The following dependencies between manipulated (independent) and controlled (dependent) variables (also referred to as effects) were considered in the experimental design:
The spark timing was set to 26.5 BTDC. The fuel used was M0 and blend M20. For Excess-air ratio (lambda) λ=1; constant speed of 2500 (RPM) and Load (BMEP) 2.4 to 7.8bars. The dependent variables included emissions of nitrogen oxides (NOx), unburned hydrocarbon, (uHC), carbon monoxide, (CO). The sampling arrangement, the sampling gas was pumped through the pre-sampler, which was connected to the gas analyzers (Figure 2) in order to measure the emissions.
Procedure and apparatus for the diesel (TDI) engine
n-butanol/diesel blend:B20 and B0 or pure diesel fuel are alternately fired in a high load, turbo-charged, diesel engine to determine the emissions characteristics using the measuring instruments as described in a similar arrangement as for the layout shown for the suzuki engine. Fuel properties such as the viscosity and solubility of additives and their mixture with diesel exert a significant impact upon the emissions produced. Although the determination and evaluation of these physiochemical properties of fuels were not intended, their underlying effects are used to interpret the results obtained throughout the study. The properties of n-butanol and DF are compared in Table 6. It should be noted that the lower heating value (LHV), and CN are higher for n-butanol than for ethanol,8 see Table 6. So it was opted to use n-butanol instead of ethanol. The engine was turbo-charged and fully equipped with all the necessary sensors and actuators for stable operation. The engine was heated until its cooling water temperature had reached 94˚C and was kept at this constant temperature. The engine was run for 20 to 30minutes to warm up with either the reference fuel DF or the test (blend) fuel B20. Once the new fuel was pumped in, the engine was made to run for another 20minutes to allow for stable operation with the new blend under test conditions. The study was carried out on full, 75%, 50% and 25% load in the operating speed of 1500RPM. The engine was made to run at steady-state condition for approximately two minutes for each measuring point before recording the values.
The results of the comparative study between the compression ignition (CI) and spark ignition (SI) engines in terms of the effects of oxygenated fuels blended with both gasoline and diesel are given and discussed in this section. For diesel propelled engine, n-butanol is the additive at 20% in diesel fuel at 1500RPM designated as b20-tdi-1500 RPM. The engine used is specified in Table 2. And gasoline operated Suzuki engine as specified in Table 1. Methanol is the additive at 20% in gasoline fuel at 2500RPM denoted as m20-suz-2500RPM.
Mass flow rate
Figure 3 and Figure 4 indicates the mass flow of fuel and effect of oxygenated blending of gasoline and diesel in both the spark ignition and compression ignition engines respectively. Since diesel always operates lean with higher compression ratio and efficiency than gasoline engine it should be as expected that the range of fuel consumption was less in the CI engine than in the SI engine. As the LHV of oxygenated fuels in this case M20, the quantity of the fuel blend is increased for the same brake mean effective pressure, BMEP. Brake mean effective pressure is a fictitious constant pressure that would give the same work cycle pressure above the piston in the combustion chamber. In the case of the diesel engine the difference in the mass flow rate is little since the CI engine is well known for its higher efficiency than the SI engine. It is reported in Ref,9 that with increasing load (Bmep) for all the fuels fuel consumption is higher for the blends than for DF. The blends have a lower heating value than diesel. This causes the engine to demand more blended fuel to be delivered than when fueled with diesel only in order to maintain the same engine-brake power output.
Emission of Nox
Thermal NOx is a by-product of combustion, and its exponential formation rate is dependent on temperature. Thermal NOx formation, which is well studied and understood,21 depends upon the residence time and higher temperatures. Figure 5 and Figure 6 illustrate the emission of Nox with Bmep for both the diesel and gasoline engines. In Figure 5 for m20, the moderate speed and probably the spark timing which was retarded to 26.5 crank angle degrees, (CAD) compensated for the short residence time and did not change much the emission of Nox with Bmep for loads of 25 and near 50%. In comparison with GF at 50% Bmep and above, the emission of Nox for the blends reduced due to improvement of brake thermal efficiency at higher loads and improved charge mixing being a multi-point injection fuel system and probably also due to the faster flame speed of the methanol –gasoline blend. When the load (BMEP) increases there is a fall in the concentration of Nox at 50 and 70% Bmep using m20 in the gasoline tests. The range of Nox emission is higher between 2000 and 4000ppm for m20 than for B20 which is from 200 to 1400ppm. The explanation for this is well known as reported as follows:7 conducted experiments on a single-cylinder, naturally-aspirated, direct-injection (DI) standard Ricardo-Cusson diesel engine. They found that the NOx emissions increase with an increasing BMEP for DF that is higher than the blends B8, B16 and B24. The reasons for these differences are explained in their paper. They believe the differences were caused by the lower calorific value, the higher heat of vaporization, and the lower CN of oxygenated butanol. However, the tests fuels by the authors were run at a low speed and the effect on Nox is depicted in Fig 6 at 1500rpm. The NOx emissions increase with an increasing BMEP for DF but are slightly lower than the blend B20 at 50 and 75% load. The engine factor (injection system) is probably the reason for the difference; in this case the authors used a turbo-charged direct injection system (TDI), which improved on the premix and diffusion controlled rapid phase combustion in the combustion chamber ref,17 raising the temperature in the combustion chamber.
Emission of unburned hydrocarbon (uHC)
Unburned hydrocarbon is a pollutant that results from an incomplete combustion of the fuel-air mixture. When the cold surfaces of the cylinder walls in a combustion chamber quench the flames, or when a UHC fuel-mixture escapes the primary combustion process, a layer of UHC is left behind, which fills the crevices (because of flame quenching at the entrance of these crevices)22 Figure 7 and Figure 8 illustrate the effect of both the diesel and gaoline engine on UHc comparing oxgenated blended fuels with their respective pure conventional fuels as used in the two engine types. The range of emissions of uHC is far less in the diesel engine than the gasoline engine.10-60ppm, 600 to 700ppm respectively. However, for M20, uHc emission reduces below that of using GF from 25% to full load in Figure 7. This is attributed to the lower carbon/hydrogen (C/H) ratio of the oxygenated fuels compared with gasoline fuel, (GF). Besides, the oxygen atoms of the blends reduce UHC emission further by more completely burning the intermediate UHC species than does GF. Also explained in ref,22 it is established that In spark ignition engines the fuel–charge is premixed and homogeneous. During engine operation this charge is maintained in a more or less stoichiometric state, where the correct amounts of oxygen is proportioned to the fuel required for complete combustion. In Figure 8 indicates the effect of the diesel engine and oxygenated blends B20 on uHC emission. The effect of oxygenated fuels is more pronounced than in gasoline engine as the diesel engine runs lean and has a higher efficiency and longer resident time for the fuel to burn in the combustion chamber than gasoline engine. However, the physical and chemical properties of n-butanol are such that the higher heat of vaporization than diesel fuel cools the combustion chamber in addition to the Lower heat value, (LHV) which impacts or diminishes the quality of uHc emissions.
Emission of carbon monoxide, (CO)
Since Carbon monoxide (CO) formation is kinetically controlled,22 the leaner the fuel-air mixture is, the slower the rate of CO formation during the primary reaction. Besides, higher temperatures in both rich and lean fuel-air mixtures ensure a dissociation of CO2 to CO in combustion products. Furthermore, insufficient amounts of oxygen to fully burn all the carbon in the fuel to a CO2 composition also cause an increase in the CO concentration. However, in the presence of an abundant oxidant (e.g. air), a more complete oxidation of the fuel results in the formation of CO2, rather than CO.21 Moreover, an air-deficient (fuel-rich) local environment generates high CO concentrations. For lower loads, the fuel-lean environment absorbs energy from the combustion products, thereby reducing the temperature which in turn slows down the CO oxidation. In hydrocarbon combustion, CO is the major intermediate species before CO2 is formed.22 Figure 9 and Figure 10 illustrates the effect of CI and SI engines and oxygenated blends on the emission of carbon monoxide. The diesel engine being a leaner fuel burning engine does reduce (about four times) the emission of CO more than the Gasoline engine. The tendancy in both cases of diesel and gasoline engine using oxygenated fuels at all test loads generally increase CO emission. In Figure 9 the formation of CO is rapid for M20 than GF. The comparative oxygen defficient of GF reduces CO formation with reference to M20. Otherwise the heat of vaporisation and cooling effect or energy dilution of the alcohol prevents the availability of the heat of reaction necessary to form CO2 from CO. In Figure 10 similarly and initially the insufficient heat of combustion to convert CO to CO2 due to the loss of energy as heat of vaporization in the primary combustion stages of burning n-butanol-diesel B20 increases CO. Altenatively, the presence of an oxidant B20 favors the formation of CO2 rather than CO.
Exhaust gases temperature, (EGT)
Figure 11 and Figure 12 shows the exhaust gases temperature, (EGT) as applied in oxygenated blends and diesel or gasoline engines. Temperatures are higher in gasoline than diesel because of the differences in the lower calorific value of the fuels. Alcohols have a lower calorific value than diesel or gasoline. The lower calorific value is lower for methanol than n-butanol. To maintain similar output power as if the hydrocarbon (HC) fuel was used, more quantity of blended fuel (M20) is needed to be injected and burned in the combustion chamber, which results in high EGT temperatures as shown in Figure 11. On the other hand, the higher heat of vaporisation of n-butanol reduces the in-cylinder temperature thereby reducing the EGT as illustrated in Figure 12.
In this study the effect of using oxygenated fuel blends in either a compression ignition or spark ignition engine was determined. A comparison was made using 20% methanol-80% gasoline, M20 with 20% n-butanol-80% diesel fuels in a naturally aspirated suzuik (66kw) engine and Turbo direct injection, (TDI) diesel engine respectively. The following results were obtained in summary.
This study was made possible through the collaboration between Hungary/South Africa Funding (UID 72384) and NRF the two facilitating universities: Tshwane University of Technology, Pretoria, South Africa and Budapest University of Technology and Economics, Budapest, Hungary; and the latter, made available the laboratory facility, Jendrassik Gyorgy Hotechnikai Laboratorium in Budapest BME where the engine experiments were conducted.
The author declares that there are no conflicts of interest.

©2019 Siwale, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.