International Journal of
eISSN: 2475-5559


Research Article Volume 1 Issue 4
1Department of Petroleum Engineering, Pennsylvania State University, USA
1Department of Petroleum Engineering, Pennsylvania State University, USA
Correspondence: Kelvin Abaa, Department of Petroleum Engineering, Pennsylvania State University, USA, Tel 8147530234
Received: September 26, 2016 | Published: December 6, 2016
Citation: Abaa K. Effect of methanol-treated fracturing fluids on multiphase permeability in tight sandstones. Int J Petrochem Sci Eng. 2016;1(4):98-104. DOI: 10.15406/ipcse.2016.01.00018
Alcohols have been widely used to improve gas production during completion of damaged wells in water sensitive formations. However, the mechanisms that govern multiphase permeability evolution using alcohols in conjunction with the variable composition of filtrate from different fracturing fluids are not fully understood. This study investigates the effect of methanol on multiphase permeability evolution in the presence of filtrate from fracturing fluids in low permeability sandstones by means of specialized core testing techniques.
The methodology employed in this study consists of three sets of experiments. The first set entails of measurements of surface tension of selected fracturing fluids at varying concentrations of methanol. The second set consists of gas displacement experiments conducted on sandstone cores initially saturated with fracturing fluid treated with methanol. Data obtained from this step include gas flow rate and pore volumes of liquid expelled from the core as a function of pore volumes of injected gas. The third set consists of effective gas permeability measurements from pulse decay techniques to obtain gas relative permeability curves.
Results show that for all fracturing fluids, the addition of methanol improves gas displacement flow rate and permeability recovery by two means; increasing the mobility of the liquid during liquid displacement by gas and increasing the evaporation of the trapped liquid after the displacement process. Additionally, it is shown that the presence of friction reducer decreases the amount of liquid expelled and suppresses the recovery of gas permeability during the evaporation of the trapped liquid in slickwater fluids. Reduction in interfacial tension upon methanol addition did not contribute significantly to improvement in gas relative permeability for all fracturing fluids tested.
This study quantifies the effect of methanol addition on rock-fluid and fluid-fluid interactions for the different fracturing fluids and determines the mechanisms that govern multiphase permeability in sandstone rocks. The obtained data is useful for model assisted analysis of post-fractured performance and to optimize fracturing fluid-additive selection to mitigate damage to aqueous phase trapping in low permeability sandstones.
Control and remediation of aqueous phase trapping are one of the most important issues that need to be addressed for efficient stimulation of low permeability sands. Maximizing ultimate gas recovery from tight sands depends on effective selection of fracturing fluid additives and design of fracture treatment prior to a stimulation operation. Wrong selection of the fracturing fluid and/or additives can end up contributing to permeability impairment. A review of literature returns numerous studies with different fluid additives and recommended practices that have been proposed to mitigate and correct damage from phase trapping. Most commonly used additives include light alcohols (methanol), surfactants and mutual solvents. Other types of treatments include Viscoelastic surfactants and foamed fracturing fluids with nitrogen and carbon dioxide gas. However, their effectiveness is limited to specific rock types at various in- situ reservoir conditions. This indicates that there is no clear cut, ideal fluid system for all formations in mitigating phase trapping and effective remediation fluids should be validated with core analysis and laboratory tests.
For successful remediation of formation damage from aqueous phase trapping, it is imperative to understand the response of the formation to the treating fluids by conducting core analysis and laboratory testing with the remediating fluid additives prior to application in the field. Laboratory tests help provide information about mechanisms that influence permeability improvement in the rock sample using the treatment additives. One important mechanism that has not been examined in previous studies is multiphase permeability evolution upon addition of remediation fluid additives.
Alcohols have been widely used to improve gas productivity during completion of wells in water sensitive formations. Barati and Liang showed that methanol is still used as a major fracturing fluid additive in the current industry, however it was McCleod & Coulter1 that first proposed the use of alcohol contained in aqueous stimulation fluids to stimulate problem wells in sandstone formations. They concluded that alcohols increase water recovery during cleanup and gas rate. Experimental studies by Abrams & Vingar2 claimed that the addition of alcohols to brine does not significantly improve gas flow rate when final drawdown in greater than existing capillary pressure gradient in the formation. However, Mahadevan & Sharma3 concluded that the addition of alcohols to stimulation treatment definitely contributes to gas productivity by reduction in interfacial tension and evaporation of the trapped water. They suggested that water removal upon addition of methanol occurs in two stages; a displacement phase where water is expelled by viscous forces and an evaporation phase that follows the displacement phase and lasts a long time.
One significant drawback associated with treatment using alcohols is that the cleanup is temporary and the well has to be retreated to improve gas flow rate. Al-Anazi et al.4 used a combination of field tests and laboratory experiments to show that gas productivity can be improved by a factor of two after treatment with methanol for the first four months and by 50% thereafter. Another major drawback associated with the use of solvent is brine precipitation associated with evaporation of water. Zuluaga & Monsalve5 demonstrated that increased evaporation upon methanol addition results in brine precipitation. This precipitation results in reduction in absolute permeability and can reduce gas productivity. A review of the published literature reveals that no work has been done to investigate multiphase permeability evolution using methanol additive with the fracturing fluid filtrate that leaks off into the rock matrix during stimulation.
In this chapter, research is focused on laboratory tests designed to determine and quantify the effect of alcohols as remediation fluid additives on multiphase permeability evolution in low permeability sandstones with the fracturing fluid filtrate. Methanol, a commonly used alcohol was used to treat selected fracturing fluids. The impact of treated fracturing fluid filtrate on multiphase permeability during fluid invasion and cleanup was investigated by means of specially designed laboratory experiments.6
Laboratory experiments in this part of the research study were focused on determining and quantifying the processes that govern multiphase permeability evolution of fracturing fluids treated with methanol to mitigate phase retention.
Experimental methodology used to investigate the remediation fluids consists of two steps:
Porous media
Samples used in this study consist of six cylindrical cores cut from homogenous blocks of the Scioto sandstone, native to the Oriskany sandstone formation in Ohio. All cores have the following properties: L= 2.5 in., ø = 7.26%, k∞ = 0.1854 md, PV = 3.79 cm3.
Test fluid systems
Three different polymer fluid systems were used to investigate the effect of methanol on multiphase relative permeability during filtrate invasion and gas flow back in sandstone cores. The fluids investigated were slick water, linear gel and borate cross linked fluid systems. Each fluid system consists of fluid mixtures with varying concentrations of methanol. Tables 1-3 present the selected fluids used for this study. The synthetic brine used to represent formation water is similar to that obtained in the Oriskany reservoir. The prepared brine had a total dissolved solids (TDS) content of 35500 ppm which contains 32 g/L of NaCl,1.2 g/L of CaCl2,0.78 g/l of MgCl2,0.31 g/L of KCl and 1.1 g/L of NaHCO3. Helium gas at room temperature was used in these experiments.
Fluid Specimen |
Base Fluid, % |
Additives |
||
|---|---|---|---|---|
Water |
KCL |
Polyacrylamide |
Methanol |
|
Fluid 1 |
95.9 vol % |
3% |
0.10% |
1% MeOH |
Fluid 2 |
94.4 vol % |
3% |
0.10% |
2.5% MeOH |
Fluid 3 |
91.9 vol % |
3% |
0.10% |
5% MeOH |
Fluid 4 |
86.9 vol % |
3% |
0.10% |
10% MeOH |
Table 1 Slickwater fluid systems with Methanol.
Fluid Specimen |
Base Fluid, % |
Additives |
||
|---|---|---|---|---|
Gel |
Water |
KCL |
Methanol |
|
Fluid 5 |
20 lb. Guar |
94.5 vol% |
3% |
2.5% MeOH |
Fluid 6 |
20 lb. Guar |
92 vol% |
3% |
5% MeOH |
Fluid 7 |
20 lb. Guar |
87 vol% |
3% |
10% MeOH |
Table 2 Linear Gel fluid systems with Methanol.
Fluid Specimen |
Base Fluid, % |
Additives |
||
|---|---|---|---|---|
Gel |
Water |
KCL |
Methanol |
|
Fluid 8 |
20 lb. Guar, 1.5 gptg Borate Crosslinker |
94.5 vol% |
3% |
2.5% MeOH |
Fluid 9 |
20 lb. Guar, 1.5 gptg Borate Crosslinker |
92 vol% |
3% |
5% MeOH |
Fluid 10 |
20 lb. Guar, 1.5 gptg Borate Crosslinker |
87 vol% |
3% |
10% MeOH |
Table 3 Cross linked Gel fluid systems with Methanol.
Surface tension measurement procedure
In this study, surface tension is obtained using a combination of capillary rise method and sessile drop method. Using the capillary rise method, a glass capillary tube placed in the test fluid will cause the fluid to rise until the weight is balanced by the vertical component of the surface tension between the fluid and the glass surface. The relationship between the height of the fluid and surface tension is given by Eq. 6.1
Eq.1
Where,
τla= interfacial tension (dynes/cm)
ϴ= contact angle (dynes/cm)
ρ= contact angle (dynes/cm)
g = gravity (980 cm/s2
r = radius of tube (cm)
ht = height of the liquid rise in capillary tube (cm)
Eq. 1 requires contact angle which can be obtained indirectly. Using the sessile drop method, the maximum height of a droplet of the same fluid that can be maintained on a glass surface using Eq. 2
…Eq.2
Where,
hd = height of the droplet (cm)
Substituting Eq. 1 into Eq. 2 few obtain:
…Eq.3
The surface tension is expressed using equation 6.4
....Eq.4
Surface tension measurements were obtained for the selected fluid mixtures at room temperature and atmospheric pressure. The inner diameter of the capillary tube is 0.0475cm. Measurements of the height of liquid rise in the capillary tube and height of liquid drop on a similar gas surface were obtained and used to calculate surface tension using Eq.4. Three measurements were taken for each fluid solution and the average was reported.
Multiphase permeability flow test
Multiphase permeability flow test consists of gas displacement experiments conducted to displace liquid from cores originally saturated with the fracturing fluid filtrate from the selected test fluid systems. The saturated core represents potential saturation conditions in invaded zone during hydraulic stimulation. The experiments were conducted in two steps. In the first step, gas displacement experiments are conducted with a specified pressure gradient over the core sample. Gas flow rate, pore volumes of gas injected and expelled liquid data are obtained in this step. In the second step, gas relative permeability measurements are obtained using pulse decay techniques at different liquid saturations of the core sample. Pulse decay permeametry is used to measure gas relative permeability. This approach minimizes capillary end effects predominant in steady state flow experiments with low permeability samples.
Core flood apparatus
The apparatus consists of a Swagelok core holder to confine the core plug at the prescribed stresses. The core holder is connected to an upstream and downstream reservoir on either side. The volumes of the upstream and downstream reservoirs were 17.36 and 3.1 cm3 respectively. Confining pressure (35 MPa) and mean pore pressure (6.89 MPa) is achieved using pressurized helium from a helium gas tank. Pressure transducers were used to monitor the upstream and downstream reservoir pressures (PDCR 610 & Omega PX302-5KGO) to a resolution of 0.03 MPa and a data acquisition system (DAS) used to obtain data collected as voltage measurements. A flow meter is connected to the downstream end of the core holder to measure the gas flow rate at the downstream end during the gas displacement. Additionally, a stainless-steel tank placed on a weighing balance is connected to the downstream reservoir to measure the weight of collected fluid displaced by gas from the core. The pumps, transducers and reservoir volumes were all calibrated prior to the start of the experiments. All measurements were conducted at room temperature. A schematic of the experimental set up is shown in Figure 1.
Core flood procedure
The first stage of multiphase permeability experiments involves determining the effectiveness of cleanup of the core sample by measuring the rate of increase of gas flow rate and liquid produced from a core saturated with fracturing fluid.
The experimental procedure for the first stage consists of the following steps:
The second stage of the multiphase flow tests involves measuring gas relative permeability using pulse decay methods. Pulse decay technique minimizes capillary end effects predominant in permeability measurements with low permeability rocks.
The experimental procedures for the second stage of measurements consist of the following steps:
The measured effective permeability to gas is normalized to endpoint permeability’s to generate relative permeability curves. Relative permeability measurements using this approach gives relatively accurate estimates of water saturation with gas permeability compared to the steady state method.
Surface tension measurements
Surface tension for slickwater, linear gel and cross-linked gel fluids were measured over range of methanol concentrations. The curves of surface tension of each fluid as a function of methanol concentration are plotted in Figure 2 with brine as base case. It also shows higher surface tension for slickwater compared to brine. High surface tension values for slickwater is attributed to polar nature of friction reducer (polyacrylamide molecules) present in the fluid which increases the adhesion tension and wet ability to the solid surface. On the other hand, values of surface tension for filtrate from linear and crosslinked gel fluids are comparable to brine. The formation of polymer cake during the leak off of process in low permeability cores yields a clear filtrate with similar properties to brine. In general, the surface tension decreases with increasing methanol concentration, thus adsorption at the gas-liquid interface.
Multiphase permeability evolution
Multiphase permeability evolution was investigated with two methods; steady state gas displacements and gas pulse decay permeability measurements. Measured data from steady state gas displacements include outlet gas flow rate, pore volumes of gas injected and pore volumes of liquid expelled. Gas relative permeability curves for various concentrations of methanol for each fluid system were obtained from pulse decay experiments.
Effect of methanol on slick water
Figure 3 presents normalized gas fluorite at the outlet end of core as a function the number of pore volumes of gas injected with varying concentrations of methanol for a slickwater saturated core.
Gas flow rate shows a steady and rapid increase for the first 200 pore volumes of gas injected. This is followed by a period of slow continuous increase in gas flow rate which progresses to about 50,000 pore volumes of injected gas before leveling out. The first period corresponds to the removal of liquid from the core by gas displacement while the second period corresponds to removal of liquid by evaporation with gas. The observed trends are consistent with the results Kamath and Laroche obtained using brine and methanol. The effect of methanol on gas flow rate becomes noticeable at about 1000 pore volumes of gas injected. Improvement in gas flow rate for 10% vol methanol is by a factor of 1.73 while moderate increase was observed for 5% methanol by a factor of 1.2. There is no noticeable difference in gas flow rate with fluid with 2.5 % methanol compared to pure slickwater fluid.
Figure 4 shows expelled liquid by gas displacement as a function of pore volumes of gas injected in a slickwater saturated core at different methanol concentrations. The liquid displacement regime is indicated by the leveling of the displaced liquid curve which corresponds to about 300 pore volumes of injected gas. This agrees with the displacement regime trend observed in the gas flow rate plot. The effect of methanol on displaced liquid is also noticeable, as the amount of liquid displaced increases with increasing methanol concentration. For pure slickwater, the maximum amount of liquid displaced is about 30% of the core pore volume. 5% volume methanol barely increases the amount of liquid displaced slightly while 45% pore volume is recovered for methanol concentration of 10%.This clearly indicates that methanol improves the mobility of the slickwater fluid which aids the displacement process.
Figure 5 presents the gas relative permeability as a function of gas saturation obtained from gas pulse decay experiments for the different methanol concentrations. There is no significant difference in gas relative permeability curves until about 30% gas saturation which corresponds to the displacement phase. This suggests that methanol does not contribute to improvement in gas relative permeability during the displacement phase. The effect of methanol becomes apparent after 30% gas saturation where there is a separation of the relative permeability curves with the 10% methanol curve showing a notable increase. This increase in gas relative permeability is attributed to the increased mobility of the liquid phase at the end of the displacement process and agrees with earlier observations from the displaced liquid curves in Figure 6. As the evaporation period progresses, the gas relative permeability eventually peaks at 90% gas saturation which corresponds to about 50,000 pore volumes of gas injected. This indicates that the increased volatility of the trapped liquid due to methanol is driving the improvement in gas relative permeability.
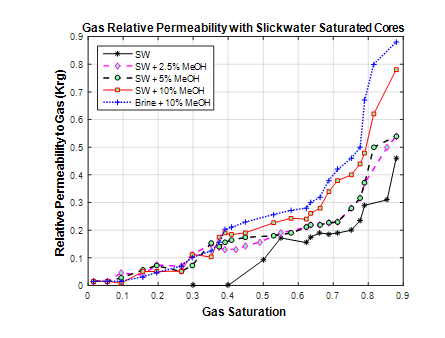
Figure 5 Relative permeability to gas as a function of gas saturation for slickwater saturated core.
Effect of methanol on linear and crosslinked gels
Figure 7 presents normalized gas flow rate at the outlet end of core as a function the number of pore volumes of gas injected with varying concentrations of methanol for a sandstone core saturated with filtrate from linear gel fluid. The profile of the curve shows the same trends observed in previous plots with slickwater; thus a displacement regime marked by rapid increase in gas fluorite followed by the evaporation regime with slow but steady increase in gas flow rate. There is no difference in the curves of pure linear gel and that containing 2.5% methanol during the evaporation regime. Flow rate improvement is observed for 5% methanol fluid during the displacement phase. Maximum fluorite improvement is obtained with 10% methanol with linear gel and is similar to brine with 10% methanol.
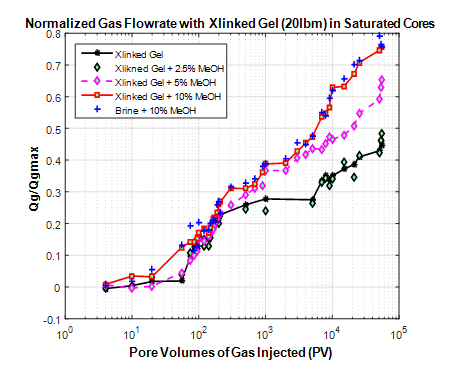
Figure 7 Normalized gas flow rate as function of pore volumes of gas for crosslinked gel saturated core.
This suggests that the leak off filtrate have flow properties similar to formation brine. Low core permeability and formation of filter cake limits polymer invasion into the core. This agrees with findings from previous multiphase permeability experiments in the literature that suggests that filtrate from fluids with natural polymer (linear and crosslinked gels) in low permeability rocks show similar properties to water. The same trend is observed for borate crosslinked fluid as shown in Figure 8
Figure 8 & 9 shows the displaced filtrate from core during gas injection. Improvement in fluid mobility is observed with increase in methanol concentration for linear and crosslinked gels respectively. The effect of methanol can clearly be seen as at the start of the displacement process marked as 5% methanol can concentration results in 50% more recovery than with pure linear gel filtrate. It may be inferred that methanol increases the capillary number by reducing the interfacial tension between the liquid and the solid phase resulting in increased liquid mobility.10% methanol concentration results in 45% pore volume of liquid expelled at the end of displacement compared to about 32 % pore volume for linear gel filtrate without methanol. Gas relative permeability curves for linear gel saturated core as a function of gas saturation is presented in Figure 10. Measured relative permeability data is obtained from pulse decay experiments for linear gel.
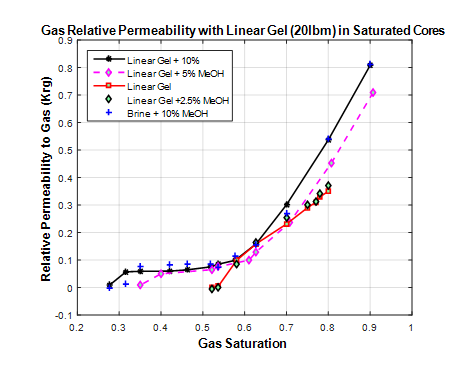
Figure 10 Relative permeability to gas as a function of gas saturation for linear gel saturated core.
The effect of methanol is clear, while there are no differences in relative permeability curves during the displacement period, the range of saturations for which there is permeability to gas increases with increasing methanol concentrations. This implies that improvement in gas permeability from methanol is not due to decrease in gas-liquid interfacial tension but to improved liquid mobility during displacement. The effect of methanol on gas permeability improvement becomes significant in the evaporation phase which corresponds to gas saturation above 70%. The same trend is observed for crosslinked gel fluid as shown in Figure 11.
This study investigates the effect of alcohol on multiphase permeability evolution in sandstone core samples flooded with filtrate from selected fracturing fluids. The fracturing fluid systems used to flood the core sample include slickwater, linear gels and borate crosslinked gel fluids. Methanol was used a remediation additive to mitigate damage from aqueous phase trapping. Multiphase permeability flow tests were conducted using steady state gas displacement methods and gas pulse decay permeametry. Experimental data include normalized gas flow rate, pore volumes of gas injected and pores volumes of liquid expelled which were obtained from steady state gas displacement tests. Relative permeability curves were generated with data obtained from gas pulse decay experiments.
The major conclusions of this study are:
cc: Cubic centimeter
cm: Centimeter
Cp: Centipoise
ft: Feet
f: Fahrenheit
fr: Friction Reducer
g: Grams
gal: Gallon
gptg: gallon per thousand gallon
in: Inches
krg: Relative permeability to gas
krw: Relative permeability to water
Kg: Effective permeability to gas
Kabs: Absolute permeability
Lb: pound
L: Liter
M: meter
mm: millimeter
mol: Moles
mD: Milli Darcy
MeOH: Methanol
MMSCFD: Million cubic feet
Mpa: Mega Rascals
psi: pounds per square inch
psia: Pounds per square inch atmosphere
pptg: Pounds per thousand gallons
ppm: Parts per million
PV: Pores Volumes
q: Flow rate
Sw: Water Saturation
Sg: Gas Saturation
SW: Slick water
Tcf: Trillion cubic feet
Vol: Volume
Ø: Porosity
%: Percent
None.
The author declares no conflict of interest.

©2016 Abaa. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.