International Journal of
eISSN: 2574-8084


Research Article Volume 7 Issue 4
1Melanoma and Skin Cancer Trials, Australia
2Sydney Medical School, University of Sydney, Australia
3Patricia Ritchie Centre for Cancer Care and Research, Mater Hospital, Australia
4Melanoma Institute Australia, University of Sydney, Australia
5GenesisCare, Mater Sydney Hospital, Australia
Correspondence: Gerald B Fogarty, GenesisCare Radiation Oncology, Mater Sydney Hospital, Rocklands Rd, Crows Nest, NSW 2065, Australia, Tel +61 2 94588050, Fax +61 2 99292687
Received: July 07, 2020 | Published: July 20, 2020
Citation: Williams N, Boyle F, Hong A, et al. Whole brain radiotherapy with partial hair-sparing technique - a feasibility study. Int J Radiol Radiat Ther. 2020;7(4):104-110. DOI: 10.15406/ijrrt.2020.07.00274
Introduction: Whole brain radiotherapy (WBRT) is a common palliative treatment for brain metastases from solid tumours. Traditionally, it is given with opposed lateral fields causing total alopecia as hair-bearing scalp skin receives the full dose. Volumetric modulated arc therapy (VMAT) can deliver WBRT with a simultaneous integrated boost (SIB) to larger metastases whilst minimising dose to critical structures such as the hippocampus. This feasibility study aimed to test the hypothesis that a reduced dose to the scalp using a VMAT hair-sparing WBRT protocol would spare scalp hair and reduce alopecia at four weeks post treatment without compromising disease control at three months.
Methods: The Hair Spare study (01.07 WBRTMel sub-study SS01.13) was an investigator-initiated, prospective feasibility study. A VMAT hair-sparing WBRT protocol was developed to limit the dose to the scalp to 16 Gray (Gy) in 15 fractions. The primary objective was the rate of alopecia at 4 weeks post RT as measured by CTCAE v4 and clinician and patient perception. Quality of life (QoL) was assessed at baseline, one month and three months post treatment with validated instruments including European Organisation for Research and Treatment of Cancer (EORTC) QoL Questionnaire (QLQ-C15-PAL+4) plus four additional questions specifically relating to hair, a visual analogue scale (VAS) to measure the perception of hair loss severity, and the total Chemotherapy-Induced Alopecia Distress Scale (CADS).
Results: Nine patients with brain metastases from melanoma (6), breast (2) and lung (1) cancer were enrolled at the Mater Sydney Hospital, Crows Nest, Australia. At 4 weeks, 5 patients were evaluable: 4 reported moderate alopecia (CTCAE v4 Grade 2) and 1 reported mild alopecia (CTCAE v4 Grade 1). All 5 wore wigs or scarves to hide hair loss. Any amount of hair loss impacted QoL. Reduced hair loss compared to complete alopecia, as usually found with conventional WBRT, did not translate to a mean improvement in QoL. There was no symptomatic intracranial progression of disease while the patients remained on study. Two patients had MRI at 3 months, and both had evidence of intracranial progression of disease within the volume that had received prescription WBRT dose. From the data collected it seems that VMAT hair-sparing WBRT was well-tolerated.
Conclusion: VMAT hair-sparing WBRT partially spared scalp hair at four weeks post WBRT and did not compromise symptomatic disease control during the study. The treating oncologists observed that the hair grew back quicker than with conventional WBRT, and that combined cytotoxic chemotherapy was additive to RT-induced alopecia. However, the study was not optimal in that data collection was hampered by patient availability. The patient population was too unwell to be followed up according to the protocol.
Keywords: radiotherapy, clinical trial, hippocampus, hair, quality of life, melanoma, brain, volumetric modulated arc therapy
Whole brain radiotherapy (WBRT) is a common palliative treatment for brain metastases (BMs) from solid organ tumours. The aim of palliative treatment is to increase quality of life (QoL). WBRT is traditionally given with opposed lateral fields.1 Hair-bearing scalp skin receives the full dose, resulting in long term alopecia and therefore decreased quality of life.2 Volumetric modulated arc therapy (VMAT) can accurately deliver conformal radiotherapy (RT) dose distributions with fast delivery times.3 VMAT can deliver WBRT with hippocampal avoidance (WBRTHA) which is associated with preservation of memory and QoL.4‒6 VMAT also allows simultaneous integrated boost (SIB) to regions of macroscopic tumour, all within the one RT course. VMAT uses megavoltage RT which is skin sparing. As VMAT treats from 360 degrees, the dose going through the scalp skin can be spread around its full circumference. As the maximum depth of hair follicles on the scalp is 4.5 millimetres (mm),7 it may be possible with VMAT to spare the first 5.0 mm of scalp skin to avoid alopecia when giving WBRT. Other groups have retrospectively reported scalp hair sparing with WBRTHA using VMAT.8 We present our experience in treating nine patients with various malignancies with a hair-sparing VMAT technique within a prospective feasibility study. The hypothesis was that VMAT hair-sparing WBRT could minimise dose to the scalp hair and therefore reduce alopecia at four weeks post treatment without compromising disease control at three months post treatment.
The Hair Spare study (01.07 WBRTMel sub-study SS01.13) was an investigator-initiated study sponsored by Melanoma and Skin Cancer (MASC) Trials and registered with Australia and New Zealand Clinical Trial Registry (ANZCTR) (ACTRN12617000507381). Ethics approval was granted by the Sydney Local Health District Human Research Ethics Committee (Reference: HREC/16/RPAH/553). Eligible patients were adults with brain metastases from any solid tumour who required WBRT, had hair worth conserving, a life expectancy of greater than four months and an Eastern Cooperative Oncology Group (ECOG) score of 0-2. The total anticipated accrual was 12 patients, including two bald control patients to assess whether the skin dose could be
The primary objective was the degree of alopecia at four weeks post treatment. This was measured using Common Terminology Criteria for Adverse Events (CTCAE) v4.0, which was current at the time. Prior to and one month after treatment, patients’ skin, hair and scalp were photographed at dermatological review and assessed for the presence of follicular ostia along with the need to wear a wig or head scarf to hide alopecia. Other objectives included brain progression-free survival assessed by routine MRI at three months post treatment and radiation toxicities using CTCAE v4.0. Quality of life (QoL) was assessed at baseline, one month and three months post-treatment with validated instruments including European Organisation for Research and Treatment of Cancer (EORTC) QoL Questionnaire (QLQ-C15-PAL+4) plus four additional questions specifically relating to hair, a visual analogue scale (VAS) to measure the perception of hair loss severity, and the total Chemotherapy-Induced Alopecia Distress Scale (CADS)9,10 to measure the impact of hair loss.
A protocol was developed with multidisciplinary input. Patients were immobilised with a thermoplastic mask in a “palate neutral” treatment position (Figure 1). Hair was placed to avoid a bolus effect, and long hair was pulled into a ponytail, exiting the immobilisation mask superiorly (Figure 2). The hairline was wired to allow for visualisation on the planning CT scan. This study was designed using a differential dose to different parts of the scalp. The scalp was divided into superior (sup-scalp) and inferior (inf-scalp) with a line 4.0 centimetres (cm) superior to the top of the pinna. The superior scalp was given a higher priority of sparing as it was thought more important. The hairline was marked with wire during planning to aid in contouring sup-scalp, inf-scalp and total scalp as organs at risk (OARs). The dose prescription to the whole brain was 30 Gy in 15 fractions. The total dose from the skin surface to a depth of 5.0 mm was kept under 16 Gy. Brain clinical target volume (CTV)11 was prescribed to 30 Gy with brain CTV V98 getting 25 Gy at least. The use of hippocampal avoidance WBRT and SIB were at the discretion of the prescribing radiation oncologist. The mean dose to the hippocampus was not to exceed 9 Gy and the maximal hippocampal dose was not to exceed 14 Gy.5 Metastases and metastatic cavities post resection were contoured as SIB boost volumes when present. SIBs were prescribed 45 Gy and four partial VMAT arcs were planned. The planning doses for OARs are summarised in Table 1.
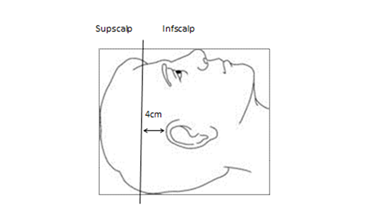
Figure 1 The scalp was divided into superior (Supscalp) and inferior (Infscalp) with a line 4 cm superior to the top of the most superior pinna out line on the planning CT with the patient lying in the “palate neutral” treatment position.
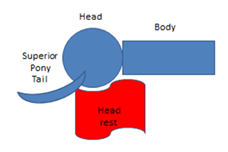
Figure 2 Hair must be placed correctly to avoid a build-up or bolus effect on scalp hair. For example, long hair could be pulled into a ponytail to exit the mask superiorly.
Left hippo |
Right hippo |
Brain CTV |
Sup scalp |
Sup scalp |
Inf scalp |
Inf scalp |
Mean < 9Gy |
Mean < 9Gy |
V25Gy > 98% |
Max< 16Gy |
Mean< 9Gy |
Max< 20Gy |
Mean< 12Gy |
Max < 14Gy |
Max < 14Gy |
|||||
Constraint met |
0/9 |
0/9 |
0/9 |
3/9 |
Table 1 Plan evaluation for OARs
CTV, clinical target volume; Gy, gray; Hippo, hippocampus; Inf, inferior; OARs, organs at risk; Sup, superior
Nine patients were enrolled at Mater Sydney Hospital over 18 months from February 2017 to July 2018. Patient characteristics are detailed in Table 2. Of the enrolled patients, eight had hair worth conserving and one was intended as a bald control to assess the delivered skin dose. After nine patients were accrued, the Trial Management Committee decided to close the study early in October 2018 due to slow accrual.
Pt |
Age sex |
Primary tumour |
No of BMs |
Hair worth Saving (Y/N) |
Previous cytotoxic chemotherapy or not (Y/N) |
Other conditions that may affect hair loss |
1 |
44F |
Breast |
4 |
Y |
Y |
Also needed cytotoxic CT within one month of RT |
2 |
67M |
Melanoma |
2 |
Y |
N |
|
3 |
46F |
Melanoma |
1 |
Y |
N |
|
4 |
42F |
Breast |
1 |
Y |
Y |
|
5 |
70M |
Melanoma |
5 |
Y |
N |
Androgenic alopecia |
6 |
61M |
Lung |
12 |
Y |
N |
Needed cytotoxic CT within one month of RT |
7 |
36F |
Melanoma |
6 |
Y |
N |
|
8 |
68M |
Melanoma |
6 |
N (bald control) |
N |
Androgenic alopecia |
9 |
72F |
Melanoma |
2 |
Y |
N |
Table 2 Patient baseline characteristics
BMs, brain metastases; CT, chemotherapy; RT, radiotherapy
RT compliance to protocol outcomes
RT characteristics are detailed in Table 3. There were significant issues with achieving the protocol. Three patients had protocol violations due to off-protocol fractionation: two were treated with 30 Gy in 10 fractions and one received 7 fractions. In no patients were the trial specific dosimetry constraints for sup-scalp met at planning. In only three out of nine patients was the mean dose for inf-scalp met. There was wide variation in in vivo dosimetry for the bald control (patient 8), but in general it was under the planned dose as seen in Table 4. The dosimetry confirmed that some scalp sparing with our technique had been achieved.
WBRT total fraction (actual) |
Number SIB in RT volume |
Numbers of hippocampi spared |
Sup scalp max/mean (Gy) |
Inf scalp max/mean (Gy) |
||
1 |
30 |
15 |
4 |
2 |
19.1/10.7 |
21.8/11.9 |
2 |
30 |
10 |
2 |
0 |
18.6/11 |
21.5/11.8 |
3 |
30 |
10 |
0 |
2 |
19.5/9.6 |
20.1/9.6 |
4 |
30 |
15 |
1 |
2 |
21.7/12.6 |
23.7/14.1 |
5 |
30 |
15 |
0 |
2 |
24/10.9 |
23.8/13.2 |
6 |
30 |
15 |
1 |
2 |
22.8/9.8 |
24.7/12.6 |
7 |
30 |
15 |
2 |
1 |
21/9.3 |
24/11.5 |
8 |
30 |
15 |
3 |
0 |
17.6/9.6 |
22.9/12.6 |
9 |
30 |
15 |
2 |
0 |
20.4/10.4 |
25.8/12.7 |
Average |
31 |
11.3 |
1.6 |
1.2 |
20.5/10.4 |
23.1/12.2 |
Table 3 Radiotherapy characteristics
Gy, Gray; Inf, inferior; Max, maximum dose; RT, radiotherapy; Sup, superior; WBRT, whole brain radiotherapy
Location |
Dose planned per fraction (cGy) |
Dose measured per fraction (cGy) |
Percentage difference |
Vertex |
47 |
46.67 |
-1 |
Midline posterior |
110 |
96.67 |
-14 |
Left 2cm superior to ear (infscalp dose) |
80 |
73.33 |
-9 |
Right 2cm superior to ear (infscalp dose) |
80 |
86.67 |
8 |
Left 2cm superior to left ear (supscalp dose) |
80 |
73.33 |
-9 |
Right 2cm superior to left ear (supscalp dose) |
70 |
65.67 |
-7 |
Table 4 In vivo dosimetry of bald control
cGy, centi Gray; cm, centimetre; Infscalp, inferior scalp OAR; RT, radiotherapy; Supscalp, superior scalp OAR; WBRT, whole brain radiotherapy
Alopecia outcomes
Hair-sparing characteristics are detailed in Table 5. Assessment of alopecia by a dermatologist was difficult as some patients were too unwell to attend a separate dermatological appointment in addition to follow up with the radiation oncologist. For the primary endpoint at four weeks, of the eight patients with hair worth sparing, five were evaluable. Three patients (patients 3-5) discontinued due to ill health. All five reported the need to use a scarf or a wig at some time after RT. Four patients self-reported moderate alopecia which was assessed as grade 2 (CTCAE v4.0) patterns of moderate hair loss. One reported mild alopecia (patient 7–Figure 4) equivalent to grade 1 (CTCAE v4.0). Three patients underwent dermatological assessment at one month post RT. One was the bald control. Two others reported significant hair loss (for example, patient 1; Figure 5) that was clinically apparent. Follicular ostia were present in these same two patients after RT. At three months, a further three patients (patients 6, 7 and 9) were lost to follow up due to clinical deterioration. Only three patients (patients 1, 2, 8) were evaluable including the bald control (patient 8). Patient 1 had severe hair loss following further cytotoxic chemotherapy, another (patient 2) had experienced some regrowth (Figure 6).
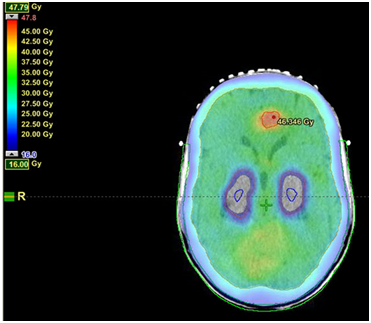
(A) Screen shot of axial plan through BM and hippocampi. Note the dose fall off as the dose cloud reaches the scalp. Doses are 45Gy to the anterior BM, 30 Gy to the whole brain with a maximum of 14 Gy to the bilateral hippocampi. Dose wash shows dose levels from 47 to 16 Gy.
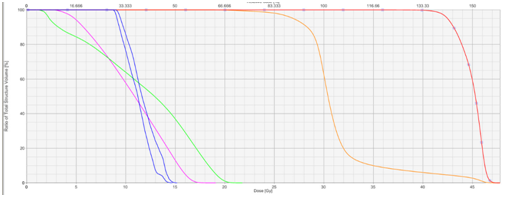
(B) Dose volume histogram of the plan.
Dose volume histograms can be constructed to ensure the tolerance doses
of the OARs are not exceeded. The X axis is the dose axis in Gray. The Y
axis is a measure of volume. The red line to the right shows the dose to the
BM. The orange line shows dose to the whole brain. The green and pink line
show dose to infscalp and supscalp respectively. The blue lines are doses to
the hippocampi.
Figure 3 Example of isodose distribution of VMAT hair-sparing WBRT with SIB for 1 brain metastasis (BM).
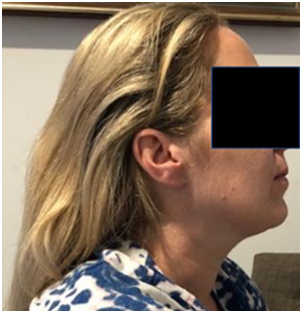
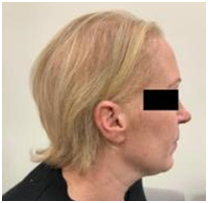
A. Patient 7 - baseline prior to RT. B. Patient 7 - 4 weeks post RT with mild alopecia
Figure 4 Comparison of hair at baseline and at 4 weeks post RT for patient 7 with no history of cytotoxic chemotherapy prior to or after RT.
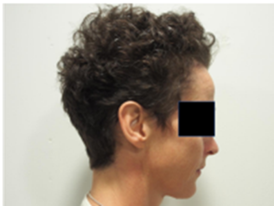
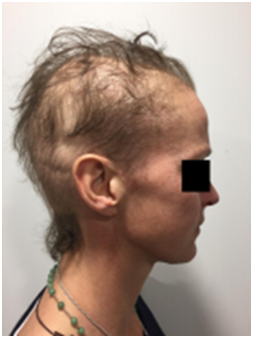
A. Baseline prior to RT B. At 4 weeks post RT with moderate alopecia
Figure 5 Patient 1,This patient had previous cytotoxic chemotherapy causing alopecia for primary breast cancer some years prior to this current course of therapy.
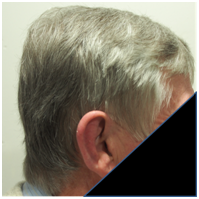
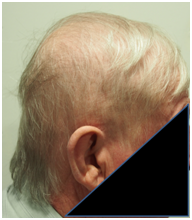
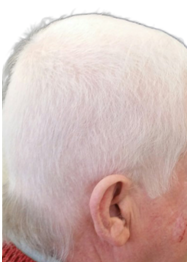
A. Prior to RT B. One month following RT C. Three months following RT
Figure 6 Chronological progress of alopecia in patient 2.
Pt |
Alopecia at 1 month |
Alopecia at 3 months |
Need for scarf/wig anytime after RT |
1 |
Moderate/Grade 2 |
Severe |
Y,had CTx after RT |
2 |
Moderate/Grade 2 |
Moderate |
Y |
3 |
Not able to be assessed |
Not able to be assessed |
Not able to be assessed |
4 |
Not able to be assessed |
Not able to be assessed |
Not able to be assessed |
5 |
Not able to be assessed |
Not able to be assessed |
Not able to be assessed |
6 |
Moderate/Grade 2 |
Not able to be assessed |
Y |
7 |
Mild/Grade 1 |
Not able to be assessed |
Y |
8 |
Not applicable (bald control) |
N/A |
N/A |
9 |
Moderate/Grade 2 |
Not able to be assessed |
Y |
Table 5 Hair-sparing characteristics: Patient self-reported as mild or moderate or severe and / CTCAE v4.0 Grade 1 or 2
CTx, chemotherapy treatment; N, no; N/A, not applicable; Y, yes
Oncological outcomes
There was no symptomatic intracranial progression of disease while the patients remained on study. Two patients had MRI at three months, and both had evidence of intracranial progression of disease. One of these had metastatic breast cancer and developed leptomeningeal disease (LMD). After reviewing the MRI against the original WBRT plan, the area of new LMD was observed not to be in the volume spared by the hair-spare technique - it had received the full radiation dose. The Trial Management Committee therefore determined that this LMD progression was not related to study treatment. This patient had an excellent response to systemic therapy and survived to have repeat courses of stereotactic radiosurgery months after the WBRT. Both patients did progress on MRI in volumes that received the prescribed WBRT dose.
Quality of life outcomes
Due to disease progression and symptoms, the QoL instruments were not fully completed as per protocol. Five patients competed QoL questionnaires at one month, but only two patients had completed the questionnaires at three months. No QoL factors were specifically different after treatment, as noted on CADS. At the one month timepoint, according to QLQ-C15-PAL+4 responses, four patients experienced ‘quite a bit’ of hair loss and one reported ‘very much’; the range on the VAS was 65-98 out of 100 (with 0 being no hair loss and 100 being most severe hair loss). Two patients were ‘quite a bit’ upset by how the treatment affected their hair at one month. At three months, both patients who completed the QLQ-C15-PAL+4 questionnaires reported hair loss as ‘very much’. Any amount of hair loss impacted QoL. Reduced hair loss compared to complete alopecia as usually found in conventional WBRT did not translate to a mean improvement in QoL. The treating oncologists also noted that the degree of alopecia was less than what would be expected with conventional WBRT that is not hair-sparing (Figures 4‒6).
Toxicity outcomes
Similar to the QoL documentation, patient illness detracted from reporting all possible toxicities. From the data collected, it seems VMAT hair-sparing WBRT was well tolerated with no treatment-related serious adverse events (SAE). There was one SAE for hospitalisation due to anxiety that was not related to treatment. There were 32 grade 1 and 15 grade 2 adverse events (AEs), as measured by CTCAE v4.0. The main grade 2 reported AEs were four alopecia, two anorexia and two fatigue (Table 6).
Adverse event |
Grade 1 (mild) |
Grade 2 (moderate) |
Hair loss/alopecia |
1 |
4 |
Anorexia |
0 |
2 |
Dry mouth/salivary gland |
2 |
1 |
Dysphagia |
1 |
0 |
Nausea |
2 |
1 |
Radiation mucositis |
1 |
0 |
Vomiting |
1 |
0 |
Skin atrophy |
2 |
1 |
Induration/fibrosis (skin/subcut. tissue) |
1 |
0 |
Radiation dermatitis |
1 |
0 |
Telangiectasia |
1 |
0 |
Myelitis |
1 |
0 |
Oedema: head and neck |
1 |
0 |
Osteonecrosis (avascular necrosis) |
1 |
0 |
Allergic reaction/hypersensitivity |
1 |
0 |
Fatigue |
4 |
2 |
Otitis external |
0 |
1 |
Ocular surface disease |
1 |
0 |
Watery eyes |
1 |
0 |
Pain |
1 |
0 |
Rash maculo-papular |
0 |
1 |
Hypotension |
0 |
1 |
Paraesthesia |
1 |
0 |
Dysgeusia |
1 |
0 |
DVT |
0 |
1 |
Fall |
1 |
0 |
Dizziness |
1 |
0 |
Other: Altered sense of smell |
1 |
0 |
Other: Difficulty remembering facts |
1 |
0 |
Other: Difficulty finding words |
1 |
0 |
Totals |
32 |
15 |
Table 6 Adverse events according to CTCAE v4.0
This feasibility study applied modern RT techniques to spare the hair and maximise QoL during WBRT with hippocampal sparing and SIB. The hypothesis was that VMAT hair-sparing WBRT would spare scalp hair and therefore decrease alopecia at four weeks post treatment. We applied a dose prescription of 30 Gy in 10 - 15 fractions. The total dose from the skin surface to a depth of 5 mm was kept under 16 Gy. Brain CTV was prescribed to 30 Gy with brain CTV V98 getting 25 Gy at least. This dose constraint was based on studies by Severs et al.12 and Lawenda et al.13 showing that the incidence of radiation-induced alopecia could be avoided or markedly decreased. De Puysseleyr et al.14 used VMAT and decreased the average median dose to the hair follicle volume to 12.6 Gy with WBRT of 20 Gy in five fractions but discontinued after 10 patients due to futility.
Accrual to this study was slow as there was a decline in the indication for WBRT during the study period based on data from well-designed randomised controlled trials.15 Some patients were also not followed per protocol due to illness. Despite this, our results show that partial hair sparing is achievable with our protocol. There was no symptomatic intracranial progression during the period of study and no grade three adverse events or treatment-related serious adverse events. Unfortunately, two patients who underwent MRI at three months had, however, evidence of distant intracranial progression in volumes that had received prescription dose.
Protocol OAR dose constraints where difficult to attain. From an RT point of view, the delivered dose may have exceeded the alopecia threshold for these particular patients. The in vivo dosimetry on the scalp surface for the bald control (patient 8) showed that the planned dose was deliverable. However, the RT dose may have exceeded the tolerance dose for hair follicle stem cells close to the medial edge of the scalp where the dose begins to increase as it comes closer to the brain CTV. The study oncologists observed that the degree of alopecia was less than what would normally be expected with WBRT, and that regrowth was quicker, especially in those not requiring cytotoxic chemotherapy post RT (Figure 4). However, there was no comparator group in the study design to enable proper evaluation. The investigators also noted that hair loss was less in those who did not have cytotoxic chemotherapy before or after hair-sparing WBRT within the study period. This raises the question as to whether the impact of cytotoxic chemotherapy on hair may be additive to the expected RT hair loss. If so, as medical oncology moves from traditional chemotherapy to targeted and immunological therapies, the efficacy of a hair-sparing WBRT protocol may be better than what was observed in the current study.
Partial hair-sparing with volumetric modulated arc therapy whole brain radiotherapy is feasible and well tolerated, does spare some hair, and and hair grows back quickly. From the data collected, it does not cause treatment-related serious adverse events, nor compromise oncological outcome.
The study team acknowledges the support of the GenesisCare team at Mater Hospital and the patients and carers who made this study possible. We also wish to gratefully acknowledge Cancer Australia support through the Priority-driven Collaborative Cancer Research Scheme (PdCCRS/1084046) and Cancer Clinical Trials Infrastructure funding towards Melanoma and Skin Cancer (MASC) Trials. Friends of the Mater Foundation are also acknowledged for supporting Professor Boyle’s involvement. Finally, we wish to thank Aileen Eiszele of A&L Medical Communications for writing assistance and manuscript preparation.
Cancer Australia supported this work through the Priority-driven Collaborative Cancer Research Scheme (PdCCRS/1084046) is gratefully acknowledged for this sub-study. Professor Frances Boyle is supported by The Friends of the Mater Foundation.
Authors declare that there is no conflict of interest.

©2020 Williams, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.