International Journal of
eISSN: 2574-8084


Case Series Volume 6 Issue 5
1St Vincent's Hospital, GenesisCare, Australia
2John Flynn Private Hospital, GenesisCare, Australia
3St Andrew’s Hospital, GenesisCare, Australia
Correspondence: Gerald B Fogarty, St Vincent's Hospital, 438 Victoria Street, Darlinghurst NSW 2010,Australia, Tel 612 8302 5400, Fax 612 8302 5410
Received: August 29, 2019 | Published: September 13, 2019
Citation: Fogarty GB, Christie D, Potter A. Volumetric modulated arc therapy (VMAT) for extended skin field cancerisation (ESFC): Radiobiological learnings from unique patient cases. Int J Radiol Radiat Ther. 2019;6(5):156-162. DOI: 10.15406/ijrrt.2019.06.00239
Patients with extended skin field cancerisation (ESFC) can have poor oncological outcomes and poor quality of life. The durability of traditional non-surgical skin field treatments is disappointing. Our national radiation oncology teams developed a technique using volumetric modulated arc therapy (VMAT) to treat ESFC which has been published. This technique was influenced by what we learned from unique patient cases, some of which are described herein. Several issues have been uncovered through our experience in treating ESFC to date: the difficulty in measuring acute skin toxicity with the current grading systems; the cause and treatment of pain during the treatment course; optimal total dose and fractionation schedules with VMAT, including the need for a treatment break; radio sensitivity of ESFC; the apparent lack of accelerated repopulation in ESFC; the impact of pre-existing immune-suppression and radiation sensitizing medications; and the need for the “spare strip”–the provision of spare strips of skin on limbs, especially the legs, to preserve lymphatic drainage. Monitoring of the success of the technique is ongoing. The technique also continues to be influenced by unique learning cases as they arise.
Keywords: actinic keratosis, basal cell carcinoma, Bowen’s disease, in-situ squamous cell carcinoma, radiation, radiotherapy, skin field cancerisation, skin neoplasms, volumetric modulated arc therapy
AK, actinic keratosis; ADLs, activities of daily living; AR, accelerated repopulation; ASCRT, adaptive split-course radiotherapy; BCC, basal cell carcinoma; Bx, biopsy; cm, centimetre; CR, complete response; cSCC, cutaneous skin field cancerization; ESFC, extensive skin field cancerization; F, female; #, fraction; Gy, Gray; IT, immunotherapy; M, male; MCpyV, Merkel cell polyomavirus; MCC, Merkel cell carcinoma; MDcSCC, moderately differentiated cutaneous squamous cell carcinoma; mm, millimetre; NMSCs, non-melanoma skin cancers; OAR, organ at risk; Pt, patient; +ve, positive; PTV, planning target volume; RT, radiotherapy/radiation therapy; SFC, skin field cancerization; SIB, simultaneous integrated boost; SXRT, superficial radiotherapy; Rx, treatment; VMAT, volumetric modulated arc therapy
Patients with skin field cancerisation (SFC)1,2 can have poor quality of life and often suffer from pruritus, flaking hyperkeratotic skin, and poor cosmesis.3 Non-melanoma skin cancers (NMSCs), including invasive cutaneous squamous cell carcinoma (cSCC) and basal cell carcinoma (BCC), can frequently arise in these fields,4 justifying their treatment.5 Patients are usually older than 60 years and may present with comorbidities that can make definitive treatment challenging. This is especially true for extensive skin field cancerisation (ESFC), defined as a field size over 50 cm2.6 The durability of commonly used topical treatments for ESFC is disappointing.5,7 New radiotherapy (RT) techniques, especially volumetric modulated arc therapy (VMAT), enable the definitive treatment of SFC8 and ESFC.6,9,10 A rind of SFC can now be homogeneously treated whilst sparing the underlying normal structures, which was not possible with previous radiation modalities.6 Treatment requires a highly trained multidisciplinary team comprised of radiation oncologists, physicists, radiation therapists and nursing staff.
With input from radiation teams from around Australia, a technique was developed to treat ESFC with VMAT.11 This technique continues to be refined based on experience and what we learn from unique cases. The issues uncovered when treating ESFC as discussed in this report include: the difficulty in measuring acute skin toxicity with the current grading systems when skin is the target; the cause and treatment of pain during treatment; radiosensitivity of ESFC; optimal total dose and fractionation schedules with VMAT, including planned treatment breaks; the apparent lack of accelerated repopulation in ESFC; the impact of pre-existing immunosuppressant and radiation sensitizing medications; and the need for the “spare strip”– the provision of spare strips of skin on limbs, especially the lower legs, to preserve lymphatic drainage. This paper describes a selection of the unique learning cases that have shaped the current protocol, particularly from a radiobiological viewpoint. Some of the findings challenge traditional radiobiological norms.
The current grading systems to measure acute radiation skin changes during RT12−14 are based on the skin being an organ at risk (OAR). In these systems, skin necrosis is graded as the most severe toxicity. In skin with SFC, there is at least in-situ cancer and skin is the target. Necrosis will be seen in this type of skin even at a low dose of RT due to the destruction of cancer tissue, which is the aim of treatment (Figure 1). Necrosis results from dying cancer cells and occurs in patches amongst areas of normal skin. Normal skin also undergoes skin reactions as would be expected in other RT scenarios, such as breast cancer. This makes the assessment and comparison of normal tissue toxicities between patients, fields and different time points challenging, especially when conducting prospective trials. This problem is also seen in Case 1.
Figure 1 shows definitive RT treatment of an ulcerated and bleeding axillary metastases of cSCC with RT prescribed to a total dose of 45 Gray (Gy) in 15 fractions (#s). After a dose of 27 Gy in 9 # given at a rate of 5 #s per week, tumour lysis is evident and result in the yellow necrotic mass that sits above the cancer mass (short black arrow). This dead cancer is already apparent at 27 Gy. Normal skin (long black arrow) inside the radiation field area marked with blue china graph pencil (short white arrow) also receives the full dose. This is because there is one cm of tissue-equivalent bolus covering the in-field skin. While the tumour is already undergoing necrosis, normal skin appears unaffected. Suitable methods for recording these concurrent forms of skin reaction are yet to be described.
Case 1
An 82-year old immune-competent male with a history of bilateral broken lower leg bones, now with venous stasis, had both legs affected by ESFC and multiple biopsy-proven (Bx) moderately differentiated cSCC’s (MDcSCC), which had previously arisen in those fields. A Doppler ultrasound scan showed good arterial flow from the bifurcation of the aorta to the feet. His legs had been heavily pre-treated by a variety of methods, including curettage. Radiotherapy was prescribed (54 Gy in 30 #s at 5 #s per week [54/30/5]) to the volume of involved skin using a VMAT technique. After 11 #s (i.e. at 20 Gy), the patient reported pain in conjunction with dry desquamation to surrounding in-field normal skin, and a yellow necrotic exudate in areas of ESFC resembling wet desquamation (Figures 2A & 2B). How to grade this acute toxicity is challenging. One way may be to separately record the normal in-field skin reaction as well as the ESFC reaction.
Some patients experience unexpected in-field pain at a relatively low dose of RT, i.e. 18-20 Gy. This is usually associated with the onset of the lowest grade acute toxicity, such as erythema in normal in-field skin and necrosis in ESFC areas, as seen in Case 1. This pain has been especially noticed early in the course of lower leg treatment. An exacerbation of pain occurs when the leg is moved from the horizontal to the vertical, especially on getting out of bed to stand up, and is presumably a gravitational effect. Patients describe a flood of pain into the irradiated leg like hot fluid coursing through the irradiated volume. This pain can impact on an individual’s independence and activities of daily living (ADLs). The pain can also be associated with swelling of the affected part. Swelling is of concern as the target is a thin rind of tissue, sometimes only one cm thick, and dermal swelling may lead to geographic miss. Swelling may also cause more dose to go to the dermis rather than the epidermis. The dermis has a higher density of vascular structures compared to the epidermis and swelling increases with dose, particularly if irradiated to a higher dose than planned. This leads to a positive feedback loop and worsening of the acute toxicity. Other problems in this scenario include whether the swollen limb will still fit into the immobilization and bolus devices designed to ensure accuracy and full dose delivery to the target skin, respectively.
This phenomenon led members of the team to suggest that a break in treatment may enable acute toxicity to subside and thus facilitate treatment completion. It was also felt to be applicable to arms as well as to lower legs. Case 1 had treatment cessation at 32 Gy in 18 #s due to pain associated with significant necrosis in the cancer bearing skin. Skin re-epithelization occurred rapidly after RT cessation (Figure 2C). The inability of some patients to tolerate the full course led the working group to advocate a break during RT consisting of at least two-weeks after 18 Gy in 10 #s at 5 #s per week (18/10/5). The break enables normal skin to repopulate before the second phase starts. This has increased the treatment completion rate. The radiobiological effect of a treatment break in SFC is discussed later in this article. Case 2 also demonstrates the pain problem, this time in a scalp.

Figure 2C Skin re-epithelization occurs rapidly. This image was taken 12 days after the image in Figure 2B.
Case 2
A 79-year-old immunocompetent male was prescribed 60 Gy in 30 #s at 5 #s per week (60/30/5) to the scalp. At 18 Gy (after 9 #s) he complained of discomfort, which was alleviated with regular paracetamol. On examination, he had erythema and a warm scalp. He insisted on discontinuing at 26 Gy in 13 #s due to pain and declined analgesia, including opiates. Three weeks after RT cessation, he was still in some pain. At three months post-treatment, all the pain had resolved, and the scalp had completely recovered with no sign of ESFC. At five months, the patient experienced in-field recurrence, indicating an inadequate dose (Figures 3A & 3B).
Some patients with NMSC seem to be highly radiosensitive and have a significantly prolonged response despite receiving a relatively low dose of radiation. In a recent series15 of 22 locally advanced lesions, 11 lesions had a complete response (CR) after just 25 Gy in 5 #s (biologically equivalent to approximately 40 Gy in 2 Gy #s given over four weeks). A review of hypofractionated RT for skin cancer in the elderly showed that approximately 40 Gy in 5 #s over two to three weeks was effective with a recurrence rate of, at worst, eight percent.16 Three case studies of hypofractionated RT in the elderly for skin cancer are also described by Veness.17 The mechanism for this deserves radiobiological interrogation. Our early experience of using VMAT in ESFC demonstrates the radiosensitivity of ESFC:
Case 3
Case 3 is a 78-year old male with a long history of multiple skin cancers. His scalp lesions were initially treated over an extended period with topical 5-FU resulting in symptomatic, painful and occasionally bleeding invasive macroscopic lesions in field. He was treated with 50 Gy in 25 #s using VMAT with no break. At the time of writing his CR was maintained at 10 months.
Case 4
The notion of radiosensitivity is also demonstrated in this case of a 59-year old male with an area of ESFC to the scalp measuring 15x12 cm. The prescription was 60 Gy in 30 #s at 5 #s per week. RT was stopped at 52 Gy due to pain needing opiates. Wet desquamation was present at that stage (Figure 4A). Follow-up ten months later revealed an in-field CR and the patient’s pain had resolved (Figure 4B).
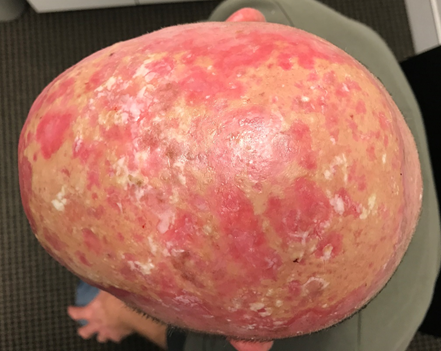
Figure 4A A 59-year old male with ESFC was prescribed 60 Gy in 30 #s at 5 #s per week. RT was stopped at 52 Gy due to pain needing opiates. Wet desquamation was present at that stage.
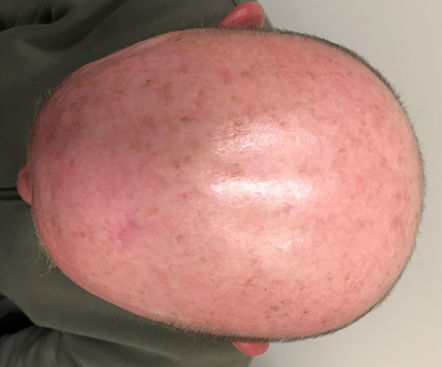
Figure 4B Ten months after stopping treatment. There was an in-field CR and the patient was pain free.
Case 5
Case 5 describes an 82-year old male with ESFC to the scalp and a long history of pre-treatment with topical and curettage. He was not immunosuppressed. The scalp was prescribed 50 Gy in 20 #s at 5 #s per week but treatment was stopped due to pain at 42 Gy in 17 #s over 3.5 weeks (Figures 5A & 5B). These cases have been tabulated in Table 1. Based on this experience, the total dose required to effectively treat ESFC with VMAT needs to be around 50 Gy. Most practitioners have since elected to prescribe 45-50 Gy in 25 #s and this is giving acceptable results in our retrospective series at 12 months.19 A dose of at least 45 Gy in 25 # would be a good starting point for a prospective study examining the in-situ component of ESFC. This dose level has been adopted in the published technique and will be revisited when efficacy data from the first prospective cohorts become available.
|
Case study/site |
Dose/#/#s per |
# size Gy |
Total dose given Gy |
**BED10 @2Gy Gy |
Result |
Comments on dose |
|
1/Lower leg |
54/30/5 |
1.8 |
36 |
38.4 |
Recurrence at 7/12 |
Too low |
|
2/Scalp |
60/30/5 |
2 |
26 |
26 |
Recurrence at 5/12 |
Too low |
|
3/ Scalp |
50/25/5 |
2 |
50 |
50 |
CR* at 10/12 |
Sufficient |
|
4/Scalp |
60/30/5 |
2 |
52 |
52 |
CR* at 10/12 |
Sufficient |
|
5/Scalp |
50/20/5 |
2.5 |
42 |
53 |
CR* at 6/12 |
Sufficient |
Table 1 Case series of in-field control with VMAT in ESFC according to total dose demonstrating radiosensitivity: (assume α/β=10 for SFC)18
*CR, complete response; **Biologically effective dose in 2 Gy equivalent does of early responding tissue using α/β of 10
The optimal total dose and fractionation schedules for treating ESFC with VMAT were defined based on our experience described above. However, the pain felt by some patients has mandated a treatment break when treating the lower legs. The radiobiological effect of a break in treatment time needs to be considered. The dose given over time for in situ cSCC is controversial. One opinion is that in-situ disease is susceptible to accelerated repopulation (AR)20 and therefore it should be treated without a break. Another opinion is that this is not the case and that a break will have no impact on efficacy. A further opinion is that a break is necessary to avoid unacceptable short-term toxicity such as pain and swelling, which may cause geographic miss (as described above with dermal swelling), and failure to complete the prescription. Our national teams’ approach, based on the types of cases already presented, is to give a two-week break between fraction 10 and 11 of a 25-fraction course. This is mandated for the lower limbs, and encouraged for head, neck, trunk and upper limb ESFC. The success of the relatively low dose of 45 Gy in 25 #s, coupled with a break of at least two weeks in limbs with SFC, has been discovered through serendipity. It seems that SFC, which is essentially SCC in situ, does not suffer from AR. This has allowed our current technique to incorporate a treatment break. VMAT for ESFC is well tolerated when given as directed above.
AR is most studied in SCC. Bese and colleagues21 have even calculated the amount of extra treatment that would be needed to compensate for unexpected breaks for SCCs of the lung, anus and mucosa of the oral cavity. However, skin cancer has rarely been included in any analysis of this type. Recent work has indicated that cutaneous SCC (cSCC) does not necessarily exhibit AR.15,22 It seems that a break in treatment of up to three weeks can be introduced to assist the healing of acute toxicities so long as there is careful clinical supervision to enable RT to be promptly restarted in the event of disease progression during the break. Our experience when treating ESFC with VMAT is that concurrent macroscopic invasive cSCCs in the field do experience AR, as demonstrated in Case 6. Other cases have not needed salvage surgery but have responded to a hypo fractionated electron boost. The clinical question needing more research is: How should an invasive cSCC within an area of ESFC be managed? Should RT still be continued to the invasive disease during the break, or can this be supplemented by an electron boost at the end?
Case 6
Case 6 is an 80-year old male with ESFC of the scalp and in-field biopsy (Bx)-proven cSCC. The patient was planned for 45 Gy in 25 #s with a simultaneous integrated boost (SIB) of 60 Gy in 25 #s with a one-month break after 11 #s (Figure 6A & 6B). The cSCC persisted at six weeks post RT. The patient was given a further 10 Gy of electrons in 2 #s to macroscopic disease. The cSCC still persisted four weeks later and was then salvaged with surgery with primary closure. The histopathology revealed an ellipse of skin measuring 43 mm x 37 mm x 7 mm containing cSCC 16 mm x 15 mm x 1.6 mm. The margins were well clear. No recurrence of ESFC was observed at 12 months post RT, and there was no recurrence of cSCC six months following surgery.
Some patients develop ESFC due to medications to treat comorbidities, such as immunosuppression.23 How do certain medications impact the use of VMAT as a treatment for ESFC? In general, from our limited experience to date, patients who are immunosuppressed, or taking medications known to be radiosensitising, are certainly more radiosensitive. This justifies the use of fully fractionated and even hyperfractionated courses. Close surveillance needs to be kept on acute toxicities as they arise. Giving hypofractionated RT by daily fractionation may result in severe acute toxicity occurring between day three and 21 after cessation of RT. RT should be titrated based on relevant patient characteristics. Case 7 illustrates several factors which can impact the delivery of RT.
Case 7
This case is of an 85-year-old male with a long history of polycythaemia rubra vera medicated for many years with hydroxyurea, a known potent radiation sensitiser. The patient wanted treatment for ESFC of the forehead which had failed previous topical fluorouracil. He had impaired mobility and wanted the least possible number of fractions. It was known that he was radiosensitive as he had been previously treated on an adaptive split course RT (ASCRT) protocol12 for a 2.0 cm exophytic cSCC on the nose, and experienced a CR after only the first phase of ASCRT during which he received 20 Gy in 5 #s with superficial radiotherapy (SXRT). His forehead was treated with 25 Gy in 5 #s over one week. After 12 months of follow-up, he maintains a CR (Figure 7).
Previous experience in irradiating limbs affected by soft tissue sarcoma24 led to the recommendation to spare a longitudinal “strip” of normal skin and subcutaneous tissue. Definitions and dose limitations for this “strip” vary (e.g. 1.0 cm width, receiving <20 Gy), and pre-date the use of highly conformal techniques such as VMAT. The rationale for this recommendation is to spare enough lymphatic channels to avoid lymphoedema. The advent of wide field RT, and the potential for it to be applied to the lower limbs where ESFC is common, raises fears about lymphoedema. Previous RT techniques would expose deep and dermal lymphatics together in one volume. When treating ESFC with VMAT, the target region is a thin “rind” of superficial tissue that includes superficial lymphatics. With careful planning, deep lymphatic channels can, however, be spared (Figure 8). VMAT can now spare the deep channels while irradiating just the dermal lymphatics. Patients with ESFC frequently have circumferential involvement, particularly in the lower limb. Identifying an uninvolved or minimally involved “strip” to leave untreated requires careful discussion with referring doctors and patients.
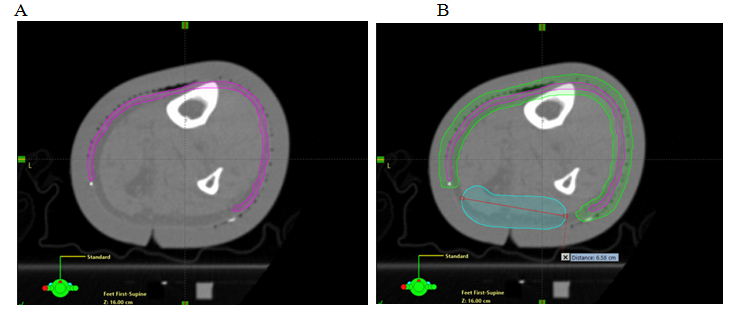
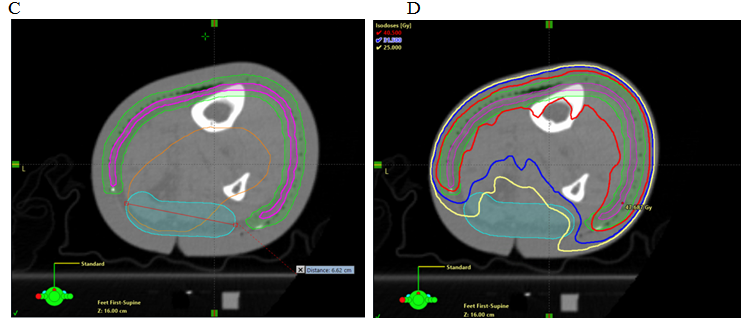
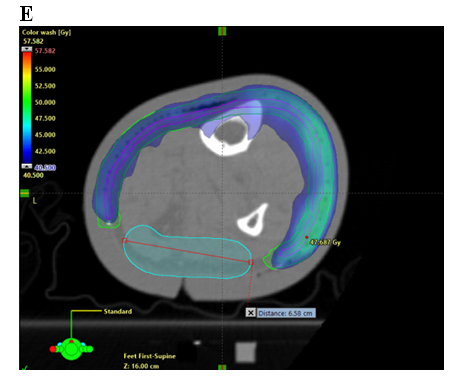
Figure 8 Sparing a strip in an axial planning CT cut of a VMAT plan. Note the sparing of deep vascular structures that include lymphatics. Axial planning CT scan of a leg showing A. Contouring of CTV; B. Contouring of PTV and strip of skin as an OAR; and C. Deep skin avoid structure added which has a dose limitation of mean less than 25 Gy D. Isodose line dosimetry showing decreased dose to strip (18-20 Gy isodose line should not be overlapping the skin sparing strip). E. Same as in D showing dosimetry in dose wash set at 40.5 Gy.
An important research question is whether it is still necessary to avoid some dermal lymphatics if the deep are spared? The minimum width of the “spare strip” remains undefined (either as an absolute measurement or a proportion of limb circumference). The most appropriate dose limit for this strip is also unclear, and it may be that sparing deep lymphatics alone allows adequate lymphatic drainage to prevent significant lymphoedema. The size of this spared area, and the dose constraint that should be applied to it, remain uncharted. For practical purposes, however, it is common that the most sun-exposed skin is on the antero-lateral side of the lower leg and the least exposed is postero-medial, so this portion of the circumference is often more suitable for sparing. As the limb narrows distally towards the ankle, the portion of the circumference needing to be treated can become greater and the tunnel able to be spared can become narrower, forming a dosimetric constriction. Until this can be better defined by reported data, our technique mandates that at least a 2.0 cm width of the circumference should remain outside the planning target volume (PTV). It should be contoured as an avoidance structure and should include at least 5.0 mm of normal tissue deep to the surface. The maximum dose should be limited to less than half of the prescribed dose and the mean dose to less than one third. In a course of 50 Gy in 25 #s, this would mean that the maximum dose in the channel would be less than 25 Gy and the mean dose less than 17 Gy. This can be more easily achieved if a distance of 5-10 mm is maintained between the avoidance structure and the edges of the PTV. Dose constraints of 18-20 Gy for this “spared” strip are reasonable but require further investigation. Without VMAT these constraints are unlikely to be achievable.
The use of VMAT in ESFC is breaking new ground. The unique cases reviewed in detail here have informed the development of our treatment protocol, but further research is needed to perfect our technique. As issues have unfolded, we have tried to distil the early learning’s from our experience to date. There is a need, for example, to correctly interpret acute skin toxicity scoring when cancer in the skin is the target. Other topics requiring further investigation include the cause and treatment of pain during treatment, the impact of pre-existing immunosuppressant and radiosensitising medications, the heightened radio sensitivity of ESFC, and the apparent lack of accelerated repopulation in this setting. Consideration also needs to be given to determining optimal total dose and fractionation schedules when treating ESFC with VMAT, including breaks during the treatment course. Finally, the recommendation to spare strips of skin on the limbs, especially the legs, to preserve lymphatic drainage deserves further research as some patients suffer circumferential ESFC and sparing a strip may be counterproductive in terms of oncological and quality of life outcomes.
The literature on total dose for in-situ cSCC is scarce and therefore controversial. In a 2012 review of seven retrospective studies, Zygogianni and colleagues25 showed that the average dose for durable control was 40 Gy (range: 10-70 Gy) in 10 to 20 #s. A diverse number of modalities and techniques, including external beam orthovoltage and megavoltage beams, brachytherapy mould and unsealed sources of radioactive paint were included. The dose per fraction in Zygogianni and colleagues.25 varied from 2 Gy per day to 5 Gy, with the latter given at a maximum of twice weekly. The authors commented that all studies showed that if the daily dose is greater than 4 Gy there is an increase in late skin toxicities, including in-field normal skin necrosis.
Some physicians in the review by Zygogianni and colleagues25 prescribed high doses of RT, similar to those for invasive disease; whereas others prescribed lower doses on the basis that the disease was non-invasive. The lower dose regimes were justified on the basis that they were treating sensitive anatomic locations (e.g. an extremity) and large treatment areas within which significant acute toxicity can arise. There was also the desire to decrease the number of fractions, and therefore visits, to the treatment facility in this older and less mobile population. In another recent review 16 of 36 relevant publications from 1983-2017 involving 12.337 irradiated lesions, the authors found that local failure in all but three of the publications was less than eight percent. The mean RT dose, mean dose per fraction, and average number of treatments per week was 38 Gy, 7.95 Gy and 2.98, respectively.
Radiobiological interrogation of the apparent radiosensitivity of ESFC may shed light on many areas of RT in cancer. Our experience to date has illustrated that VMAT for ESFC provides an opportunity to refine our radiobiological approach to in situ cSCC. Forty-five Gy in 25 fractions at five fractions per week (45/25/5) with a two-week break after the first ten fractions, and mandated for limbs, is our current technique and this is working. This schedule ensures completion of treatment without excessive acute toxicity. At this stage, we consider it safe to include a treatment break as in situ cSCC has not exhibited accelerated repopulation. More follow-up and greater patient numbers are, however, needed to assess long-term oncological outcomes. Prospective trials using this technique will generate data to further refine the use of VMAT in ESFC.
Presented at the Australasian College of Dermatologists 2019 Annual Scientific Meeting, Melbourne 18-21 May 2019; submission number 9980. The authors would like to thank Aileen Eiszele of A&L Medical Communications for writing assistance, manuscript preparation, and for over-seeing the submission process.
None.
Nill.
Author declares that there is no conflict of interest.

©2019 Fogarty, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.