International Journal of
eISSN: 2574-8084


Research Article Volume 7 Issue 1
Radiodiagnosis Department, Faculty of Medicine, Menoufia University, Egypt
Correspondence: Ashraf Anas Zytoon, Radiodiagnosis Department, Faculty of Medicine, Menoufia University, Menoufia, Egypt, Tel 00201000219818
Received: February 16, 2020 | Published: February 24, 2020
Citation: Elmageed MKA, Alwahed MSA, Zytoon AA. Ultrasound and CT guided biopsy of suspicious musculoskeletal lesions: diagnostic performance and implications for the management. Int J Radiol Radiat Ther. 2020;7(1):16-22. DOI: 10.15406/ijrrt.2020.07.00258
Purpose: Image-guided musculoskeletal (MSK) biopsy could afford initial and ultimate judgment and lead choices to the best treatment. Nonsurgical intervention grants less complication rate than surgical intervention. It is rapid, harmless and decisive procedure yields accuracy in diagnosis up to 96%. Ultrasound and computed axial tomography represent the key procedures in performing MSK intervention. Numerous needles and procedures are existing for helping the physician accomplishing this procedure without harm. This study was accepted in order to evaluate ultrasound and computed tomography (CT) guided core needle biopsies (CNB) of musculoskeletal lesions accurateness, and medical worth in the management plan.
Materials and methods: Fifty patients with MSK lesions underwent image guided (Ultrasound or CT guided) CNB for histopathological diagnosis. Radiological, pathological and clinical data were recorded and analyzed.
Results: Of fifty CNBs, forty nine (98 %) were adequate to obtain pathological diagnosis while one CNB (2 %) seemed to be inadequate. Of the fifty CNBs, forty eight patients (96%) were correctly diagnosed while two patients (4%) were misdiagnosed and this gives an overall accuracy rate of 96%.
Conclusion: The image guided CNB of MSK lesions signify the decisive and harmless method for gaining a final histopathological decision.
Keywords: biopsy, core needle, image guided, musculoskeletal, Ultrasonography
CT, computed tomography; CNB, core needle biopsy; MRI, magnetic resonance imaging; MSK, musculoskeletal; PCNB, percutaneous core needle biopsy
Percutaneous core needle biopsy (PCNB) is a reputable, extensively used, cheap technique to investigate musculoskeletal pathology.1 According to published literature, PCNB has high-impact over surgical biopsy as PCNB technique is easy, not as much pain, with rarer complications, and regularly could be achieved in the outpatient clinics under local anaesthesia.2 The close cooperation of a multispeciality team; interventional radiologist, orthopedic surgeon, medical oncologist, and professional musculoskeletal (MSK) pathologist is very critical.3 The goal of our study is to demonstrate the clinical practice and procedure of MSK biopsy and to prove its precision and safety of PCNB in different musculoskeletal pathology.
At Radiology department of our university hospitals 50 successive image guided CNBs had done for deeply located musculoskeletal lesions,
The Indications for PCNB were:
The only two contra-indications were; bleeding diathesis and decreased platelet count.4 As an over-all principle, gaining tissue-sample was requested when it would have an impact upon patient management strategy (Figure 1). Any musculoskeletal lesion could be surely analyzed as ‘‘benign’’ on imaging and clinical data (e.g., ‘‘Do not touch lesions’’) should not be biopsied,5 instead follow-up is advised in cases where the appearance does not match with the classic radiological criteria and there is no aggressive criteria. Our patients had been evaluated by a medical oncologist and surgical oncologist together with other medical team from; radiology and pathology departments. Patient’s laboratory data, including coagulation profile and platelet count were checked. Other imaging findings cautiously revised. Other imaging investigations were requested when necessary, and the procedure was postponed until the required imaging investigations were achieved and optimum. The function of radiology is not restricted to detect MSK lesions, but more significantly, embraces staging of bone and soft tissue tumors that shall benefit physicians to adopt the ideal treatment. Incidence of distant tumor spread could be regularly cancelled operative therapy, and the biopsy yet is significant as the histopathology could adopt with the appropriate chemoradiation treatments.3 After assessment of the required data, we chose the path and radiological access used to guide biopsy. Most of musculo-skeletal biopsies at our hospitals are done under ultrasound guidance.
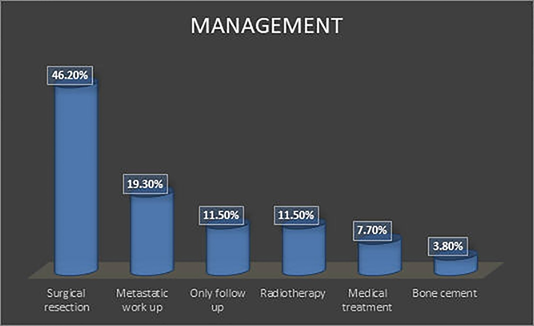
Figure 1 Bar chart shows impact of bone biopsy on the management strategy.
Note: Most of the patients underwent surgical resection (46.2%) while the remaining cases underwent metastatic work up (19.3%), medical treatment (7.7%), radiotherapy (11.5%), Bone cement fixation (3.8%) or follow up only for benign lesions (11.5%).
The path should be securely avoiding vital, healthy neurovascular bundles. The straight route to the pathology could be not fundamentally the ideal pathway. Overall, try to avoid the path that has more than one anatomic partition would be significant to protect a limb-saving operative strategy, considering that the biopsy-needle pathway might represent a hazard in spreading cancer.6,7 Since the needle path might be resected, the tissues that are located along the pathway might be eliminated. Diminishing partitional tumoral corruption is thus vital, to afford near vital organ function post-operatively. For instance, if any lesion in the skeletal system has an exophytic part which may include for example the lateral vasti group, then the biopsy path should not cross the rectus femoris or patellar tendon (only one anatomic unit as far as possible) as these might be resected throughout surgery, so the patient may be deprived of the knee extension dynamics.8 A venous access (18-20 Gauge) peripherally adopted must be attained earlier than the procedure, to ensure a fast intravenous entrée in case medicines or transfusions are mandatory.4 Utmost bone biopsies are achieved with good local anesthesia to afford ease throughout and later after the biopsy. General anesthesia is frequently needed for patients who cannot endure motionless and children.9
Ultrasound is the regular procedure used for accomplishing soft tissue biopsies. Ultrasound extensively exists, negligibly aggressive, real-time and multiplanar modality. The great dimensional and contrast discrimination similarly permit effortless imagining of lesions, chiefly minor lesions problematic to anticipate on non contrast computed tomography. While CT or MRI could be utilized, the dynamic complexity of the imaging permits simple and convenient medical team and patient positioning, and the live-imaging of the biopsy set affords comfort having harmless, the suitable biopsy pathway could be achieved and so crucial viscera are bypassed. On using the ultrasound guidance the following steps are adopted; firstly informed consent and revising the coagulation profile of the patient were done. The biopsies were performed under real-time sonographic guidance using either 5-12 MHz linear array probe or 3.5-5.1 MHz convex probe, the choice between them depended on the site of the lesion wither it's superficial or deep. The needles used in the core biopsy were the TRU-Cut biopsy needle, 14-18 Gauge, 100-200 mm long, and bone biopsy needle. After proper exposure of the area of interest, place the patient to make the lesion accessible to the examiner for scanning by ultrasound and for biopsy taking. Scans were obtained with the patient lying flat on his back, flat on his abdomen, decubitus, or semi-sitting situations. After all measurements were obtained, color Doppler sonography was used to accurately localize the lesion, to define its relation to adjacent vascular structures, to select an optimal biopsy route, and to avoid necrotic areas. After the size and location of the lesion were recorded; skin disinfection was done using Betadine disinfectant and alcohol. Then, sterile gel was applied to the optimal biopsy route; and local anesthesia (2% lidocaine) was administered under ultrasound guidance, then a stab incision was achieved with a No. 11 blade. The selected cutting needle is introduced parallel with the ultrasound transducer longitudinal plane to assist real-time imaging of needle introduction. At that time the biopsy needle is introduced along the same path as the formerly injected local anesthetic needle (Figures 2&3). Two to three cores were attained and preserved in 10% formalin for histopathology.
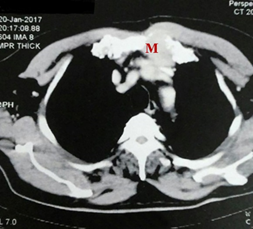
Figure 2A Contrast enhanced CT image axil cut of anterior chest wall sternal lytic lesion with soft tissue component in a 61 years old male with chest wall mass.
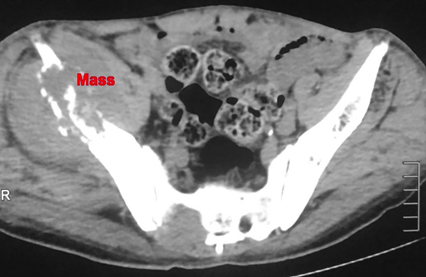
Figure 3A Contrast enhanced CT scan axial cut of right iliac bone osteolytic lesion in 70 years old male.
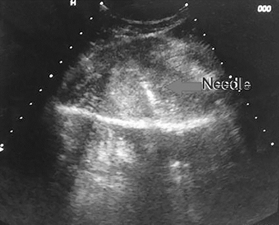
Figure 3b Ultrasound guided biopsy from the lesion, histopathology revealed the lesion to be metastatic.
The biopsy specimen was then sent to the pathology lab where it was subjected to the following: first it was fixed in a formalin solution 10% for 24 hrs, then it was applied to tissue processing for dehydration, cleaning and infiltration by the following materials (formalin, alcohol, xylene, soft paraffin, and hard paraffin), then it was stained by hematoxylin and eosin stains and finally revised by the pathologist. At the completion of the procedure, patients were observed for a minimum of 2 hours to ensure hemodynamic stability. For each biopsy, the duration time from placement of the patient on the intervention table to completion of the procedure and placement of a bandage on the puncture site was recorded. It ranged from 20 to 30 minutes. The success of each procedure was established at a review of the final pathology or the cytology report when appropriate. The location and size of the lesion, the number core) and type of the needle, the procedure time, the diagnostic yield, and any complications were recorded. On using CT guided and as for ultrasound guided biopsy, all patient's previous imaging studies are reviewed. Then the coagulation profile is checked and informed consent is obtained. Meanwhile, Selection of the proper core biopsy needle is done. A CT level is selected that portrays the lesion and a free biopsy pathway from vital structures. Occasionally it isn't likely to maintain a deliberate distance from internal viscera such as intestinal loops, so fundamental is to utilize a thin-gauge needle through.
There is no proof of adding risk of infection or bleeding after passing through those organs. The cutaneous metallic marker is used and the needle is introduced to a predecided deepness and route. Needle visualization with CT is outstanding, especially if the needle is placed ideally matching with the X-ray central beam. On the other hand, in the event that the needle is redirected cranially or caudally to be closer to the lesion, numerous additional CT scans will be required to re-image the new location of the needle tip. The needle terminal end is regularly associated with a black artifact in the contiguous soft tissues whilst in the CT scan over the needle shaft is dissimilar from the needle tip: the shaft has a tapered rather than an abrupt appearance with no associated artifact. Once the needle tip is checked inside the desired musculoskeletal lesion, the needle is fired and a core biopsy is obtained (Figures 4&5). We usually obtain at least 2-3 cores to be referred to histopathology in 10% formalin. The following histopathological steps are followed as for that of the ultrasound guided biopsy core. Post biopsy follow up is also the same as for ultrasound guided biopsy.
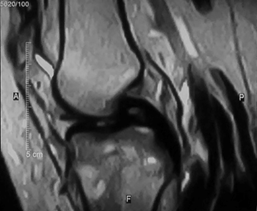
Figure 4A MRI T2WI image showing proximal right tibial intramedullary lesion with heterogeneous signal intensity and marrow edema associated with cortical irregularity, in 16 years old female.
Fifty patients (M=28 and F=22) were referred to our department for image guided biopsy from musculoskeletal lesions. Forty patients were selected for sonographically guided biopsy while ten patients were selected for CT guided biopsy. The image guided biopsies were forty (bony) and ten (soft tissue) lesions (Table 1). The lesions arise from different regions in the body. Only nine were multiple at the presentation and the remaining forty one were single at presentations. The radiological features of the lesions differed a lot. As regards the pattern of the lesion; twenty three were lytic, six were sclerotic, eight were mixed and thirteen were of soft tissue density. But regarding the consistency only four were cystic, forty four were solid and two were mixed (Table 2). The periosteal reaction was present in six lesions, we avoided taking a biopsy from the periosteal reaction alone and instead we have taken biopsy from the true lesion as well as the periosteal reaction.
Type of lesion |
Site |
Frequency |
Valid percent |
|
Soft tissue lesions |
Calf muscles |
1 |
10% |
|
Intercostal muscles |
1 |
10% |
||
Leg muscles |
2 |
20% |
||
Paravertebral muscles |
1 |
10% |
||
Subcutaneous tissue |
2 |
20% |
||
Thigh adductor muscles |
2 |
20% |
||
Gluteal region |
1 |
10% |
||
Total |
10 |
100.00% |
||
Bone lesions |
Long bones |
Diaphyseal |
8 |
20% |
Epiphyseal |
10 |
25% |
||
Metaphyseal |
6 |
15% |
||
Flat bones |
Iliac bone |
6 |
15% |
|
Rib |
5 |
12.50% |
||
Scapula |
2 |
5% |
||
Vertebral |
3 |
7.50% |
||
Total |
40 |
100.00% |
||
Table 1 Anatomical distribution of soft tissue or bone lesions
Variable |
Frequency |
Percent |
|
Radiological pattern of lesions |
Lytic |
23 |
46% |
Mixed |
8 |
16% |
|
Sclerotic |
6 |
12% |
|
Soft tissue |
13 |
26% |
|
Total |
50 |
100.00% |
|
Radiological consistency of lesions |
Cystic |
4 |
8% |
Mixed |
2 |
4% |
|
Solid |
44 |
88% |
|
Total |
50 |
100.00% |
|
Associated periosteal reaction |
Absent |
44 |
88% |
Present |
6 |
12% |
|
Total |
50 |
100.00% |
|
Soft tissue component |
Absent |
29 |
58% |
Present |
21 |
42% |
|
Total |
50 |
100.00% |
|
Table 2 Radiological characteristics of lesions
The needle size used, ranges from 14 to 18 gauges where the 18 gauge has been the most frequently used. Adequacy of the sample, evaluated by the satisfaction of the pathologist, was adequate in forty nine lesions and inadequate in only one lesion which needed re-biopsy (Table 3). The post procedure follow up revealed no definite major complication related to the biopsy. The histopathological results were recorded and revealed different pathological diagnosis of the lesions (Table 4). Overall diagnostic yield at that point would be established on the basis of accurately diagnosed cases. The case is considered correctly diagnosed on three bases; if the tissue sample gained by CNB was adequate to establish the histopathological diagnosis and match the surgical final histopathological result or if the lesion diagnosed as metastasis show size regression after successful chemotherapy or radiotherapy, or if the lesion diagnosed as benign shows stationary course on serial follow up imaging. On the other hand, the case is considered misdiagnosed in the event that the tissue sample obtained by CNB wasn't adequate to set up a conclusive histopathological diagnosis or the surgical final histopathological result didn't meet the CNB histopathological result (Table 5).
Variable |
Frequency |
Percent |
|
Radiological tool guiding biopsy |
CT |
10 |
29.90% |
US |
40 |
70.10% |
|
Total |
50 |
100.00% |
|
Needle size |
G18 |
22 |
44% |
G16 |
15 |
30% |
|
G14 |
13 |
26% |
|
Total |
50 |
100.00% |
|
Adequacy of tissue sample |
Inadequate |
1 |
2% |
Adequate |
49 |
98% |
|
Total |
50 |
100.00% |
|
Table 3 Details of the sampling procedure
Variable |
Frequency |
Percent |
|
Histopathological result of needle biopsy |
Metastatic lesion |
13 |
26% |
Osteomyelitis |
5 |
10% |
|
Osteosarcoma |
4 |
8% |
|
Giant-cell tumor |
3 |
6% |
|
Neurogenic tumor |
3 |
6% |
|
|
Ewing sarcoma |
3 |
6% |
Shwannoma |
2 |
4% |
|
Lymphoma |
2 |
4% |
|
Non-ossifying fibroma |
2 |
4% |
|
Simple bone cyst |
2 |
4% |
|
Lipoma |
2 |
4% |
|
Aneurysmal bone cyst |
1 |
2% |
|
Bone malignancy for immunostaining |
1 |
2% |
|
Chondroblastoma |
1 |
2% |
|
Enchondroma |
1 |
2% |
|
Eosionphilic granuloma |
1 |
2% |
|
Insufficient biopsy |
1 |
2% |
|
Malignant epithelial neoplasm |
1 |
2% |
|
Myosarcoma |
1 |
2% |
|
Myxoid spindle cell tumor |
1 |
2% |
|
Total |
50 |
100.00% |
Table 4 Histopathological result of needle biopsy
Frequency |
Valid Percent |
|
Correctly diagnosed |
48 |
96% |
Misdiagnosed |
2 |
4% |
Total |
50 |
100.00% |
Table 5 Over all Accuracy Rate of needle biopsy
There are limited amount of prospective randomized data in musculoskeletal interventional procedures,10 so a substantial prospective evidence in the field could add more potential benefits from the added cumulative experiences. For a historical documentation and appreciation, the initial bone biopsy was achieved by Ellis, 1947.11 In spite of the improvement in non-invasive medical imaging (radiographs, ultrasonography, CT, MR), outcomes could be yet not decisive and material from a lesion (from an image guided percutaneous or open surgical biopsy) is still necessary to judge upon its benign versus malignant nature.12 The best technique and the best imaging guide are not universally accepted and this raised a controversy in the scientific community on different approaches of biopsy taking on musculoskeletal disorders so more studies are needed to reach technique standardization.13 The planned biopsy location ought to be scanned carefully to imagine all critical anatomic structures within the range. The ideal biopsy path should be decided based on the recommended anatomical guidelines for MSK biopsy by avoidance of the nearby vessels/nerves, and escaping the muscles if probable and if malignancy is suspected, consultation with orthopaedic oncologist is imperative to appropriately plan the biopsy tract to avoid subsequent failing to ensure limb salvage surgery.14
Our results comply with previous works by previous literature in the field which recommended that ultrasound is the best procedure with the proven efficacy of MSK lesions as a prime choice rather than CT guided biopsy due to higher cost, ionizing radiation exposure, longer time and other disadvantages, and open biopsy with more complications, higher costs and greater strains in patients.1−9,11,12,15 Ultrasound is simple to handle, and provides multi-dimensional, real-time procedure, is typically faster than any other guiding procedure. Ultrasound has no radiation hazards, and is perfect in soft-tissue or bony lesions with cortical destruction. Despite all these merits, we should be careful about depending upon ultrasound alone as that choice is influenced by equipment availability, patient anxiety, and radiologist expertise and preference. In our practice, we favor ultrasound as the best procedure. Yet, following prior studies that adopted CT guidance as a method of choice we consider CT guidance as the preferred method for performing percutaneous CNB if the lesions we were asked to biopsy were not visible on the sonogram and if technical worries are expected (e.g., deep paraspinal lesions, air artifacts present). Our skills with the accurateness of percutaneous core needle biopsy is reliable with earlier reports in pediatric and adult age groups which showed correctness percentages from 74% to 94% with our work achieving a total average accuracy rate of about 91%, that is why we recommend for our orthopedic oncologists, MSK image-guided biopsy rather than open biopsy for convenience and cost containment.1−3,12,16−23
There are several factors related to the failure of the procedure so it is crucial to learn how to avoid the factors lead to non-diagnostic (ND) results after image-guided biopsies of MSK lesions that can postpone conclusive diagnosis and treatment. For justification, all procedures were performed by interventional radiologists with 15-25 years’ experience in interventions, so our higher accuracy rate highlight the significance and advantages of referring patients with a probable musculoskeletal swelling to a specialized interventional referral center as the diagnostic accuracy rates in the current study are likely partly attributable to the expertise of our interventional radiologists and pathologists and the efficient communication between the MSK oncology team members. As it should be noted that tumors of the musculoskeletal system should only be biopsied by an interventional radiologist trained in orthopaedic oncology to decrease the diagnostic errors and complication rates (i.e. the need for wider tumor resection at the time of surgery, skin complications requiring flap coverage, increased risk of amputation).24 There are major related factors of controversy about the accurateness, success/failure of image guided biopsy of MSK lesions. In the current study, we believed in core needle biopsy (CNB) over fine needle aspiration cytology (FNA). Although FNA can be less traumatic and less harmful, it only provides cellular information and tissue architecture couldn't be inspected, so we considered CNB as the gold stander for MSK biopsy, and this is consistent with most of the previous studies.25
It is indispensable to focus the attention of the interventional radiologist about highly vascular lesions that may produce only blood in the aspirate, the extensively necrotic lesions that can produce no viable tissue and lymphomatous lesions that often have crush artifact on the core biopsy specimen, precluding a definitive diagnosis. Although previous studies suggested that the diagnostic yield of CNB of MSK lesions was increased with the number of specimens obtained 26, our work results revealed that three cores were enough to obtain the pathological diagnosis and this is consistent with the results obtained by other researchers who suggested that the diagnostic yield of CNB of MSK lesions was augmented with the quantity of samplings gotten, with a plateau noted at three obtained specimens.12,26 Although the previous studies postulate that the extraosseous component of malignant bone tumors is as representative of the tumor as is the intraosseous bony component, We always avoided taking biopsy from the periosteal reaction or extraosseous component alone and instead we took biopsy from both the intraosseous and extra-osseous components.27 The biopsy tract was a major issue of concern in our study, taking into consideration the newly introduced "limb sparing/salvage surgery" in management of malignant MSK lesions. By using this new strategy; we minimize the risk of tumor seedling and orthopedic oncologic surgeons can remove bone sarcomas without amputation in the vast majority of patients, with only a limited loss of limb function.28
The needle size is another matter of controversy, while a lot of the previous studies suggest the diagnostic accuracy increases with increasing the needle gauge.20,29 We agreed with Hryhorczuk et al.15 that in the presence of well-trained MSK specialized pathologists, even small gauge specimens yields accurate pathological diagnosis, With our study achieving diagnostic accuracy of about 91% with needle size ranging from 16 to 20G.The smaller gauge guarantees lower risk of complications. Our complication rate was 0%, and this is consistent with the previous study done in both adult and children age groups which documented complication rate ranging from 0 to 8%.1,3,19 We recommend for the lesion located within the bone, then a larger (i.e. 14-gauge) cutting needle ought to be used to permit for bone fragments to be contained within the needle sample and if the lesion situated within the soft tissues then a minor (18 or 20 gauge) needle is usually adequate, that match with the previous studies.30−36 While a lot of the previous studies suggest the diagnostic accuracy increases with increasing the needle gauge. Our results proved that in the presence of well-trained MSK specialized pathologists, even small gauge specimens could yield an accurate pathological diagnosis. With our study, we achieved diagnostic accuracy of about 91% with needle size ranging from 14 to 18G. All our biopsy procedures were performed using generous local analgesia along the entire anticipated biopsy tract (including periosteum and adjacent muscles) preceding the initiation of the procedure as sedation if utilized necessitates supplementary monitoring (i.e. vital signs and pulse assessment) and personnel (i.e. anesthesiologist).10
This study is limited by a very small cohort of patients and further research with larger sample should focus on the crucial value of MSK image-guided biopsy skills. Also, MRI is well known for better identifying and avoids areas of necrosis, but unfortunately we did not have cases used MRI-directed to obtaining biopsies.
Percutaneous biopsy guided by ultrasound and computed tomography from MSK lesions can maximize patient safety, promote procedural productivity, and optimize clinical outcomes.
None.
None.
Author declares that there is no conflict of interest.

©2020 Elmageed, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.