International Journal of
eISSN: 2574-8084


Case Report Volume 6 Issue 4
General Hospital of Kymi “Georgios Papanikolaou”, Greece
Correspondence: Elisavet Glynou, General Hospital of Kymi “Georgios Papanikolaou”, Greece, Tel 6937221322
Received: June 12, 2019 | Published: August 7, 2019
Citation: Glynou E, Maria C, Falireas V, et al. The use of ultrasound in diagnostic imaging of nafld and the ultrasonographic staging in rural region hospitals. Int J Radiol Radiat Ther. 2019;6(4):135-138. DOI: 10.15406/ijrrt.2019.06.00234
The purpose of this clinical paper is to discuss the use of ultrasound technology in diagnosing NAFLD as well as to highlight the ultrasound findings and to categorize them. In this study a convex transducer 4C MHz and a General Electric Logiq P6 Pro ultrasound machine were used. Out of the total 1150 patients examined as well as 7 obese children, a total of 582 patients were diagnosed with NAFLD. In conclusion, ultrasound imaging is an effective way of diagnosing NAFLD as well as differentiating between the different stages of the disease.
Keywords: ultrasound, Non-alcoholic fatty liver disease, echogenicity, fatty infiltration
Non-alcoholic fatty liver disease (NAFLD) is the most common diffuse liver disease, with a worldwide prevalence of 20% to 46%. NAFLD can be subdivided into simple steatosis and nonalcoholic steatohepatitis. Most cases of simple steatosis are non-progressive, whereas nonalcoholic steatohepatitis may result in chronic liver injury and progressive fibrosis in a significant minority. Fatty infiltration of the liver is the result of increased deposition of fat in the liver, which amounts to 5% or greater of the organ’s weight.1 The most important causes are: obesity, type 2 diabetes, toxins and medication (mainly hydrocortisone). It is histologically classified into either macrovesicular or microvesicular steatosis based on the size of the fat droplets accumulated in the cytoplasm of the hepatocytes. During the last 20 years, prevalence of NAFLD has doubled probably due to the increased prevalence of overweight and obesity in the paediatric population worldwide.2−4
Our study’s main purpose is to highlight the importance of U/S in diagnosing NAFLD, especially in rural areas or islands where the use of CT tomography or MRI is difficult, as well as showcasing the ultrasonographic staging of the disease.
In a 4-years period (from 2016 to 2019) a retrospective study of 887 patients with fatty infiltration of the liver was performed, out of which 608 were males and 272 were females, aged 42 to 69 and 7 obese children (age from 8 to 13 years). All patients were examined in the ultrasound cabinet of our hospital (hospitalized, regular appointments and emergency incidents) and subjected to upper abdominal ultrasound scan, with convex transducer 4C MHz, in three ultrasound imaging planes: transverse, oblique and sagittal planes.5−7 In order to better depict the liver, especially in obese patients or those with excessive intestinal gas, the patients were asked to inhale deeply and hold their breath for the duration of the ultrasonographic examination for a few seconds at a time, so that the liver could be visible in its entirety. The ultrasound examination revealed the echogenicity of the parenchyma of the liver and was compared with the parenchyma of the right kidney, the spleen and the pancreas (Figure 1−3). Τhere is always the risk of missing a diagnosis of mild hepatic steatosis on ultrasound if there is concurrent chronic renal disease, which increases the echogenicity of the kidneys (Figure 4), if there is any doubt that the patient might have a chronic renal disease, comparison of the liver to the left kidney and the spleen may be useful.8
The fatty infiltration of the liver is shown on ultrasound with significantly increased echogenicity "bright liver", in comparison with the right kidney cortex (Figure 1). Normally, the liver and the renal cortex have similar echogenicity, relative to the parenchyma of the pancreas and the spleen. The maximum diameter of the liver in the midclavicular line (MCL) was increased, right lobe>15 cm (Figure 5). Also significant features are the absence of mass effect on intrahepatic vasculature (Figure 6) as well as the poor visualization of the deep parts of the liver (Figure 7), due to decreased permeability of the acoustic beam. A focal or diffuse morphology has been shown to increase the echogenicity of the fatty liver. Differential diagnosis is caused by focal fat infiltration (Figure 8), which has a geographic distribution. In this case, hypoechoic areas correspond to normal hepatic parenchyma on fatty infiltration sites, whereas, normal parenchyma islets can easily be identified due to their typical positions, in front of the right branch of the portal vein, its division, around the area of the gallbladder and the caudate lobe.9−13
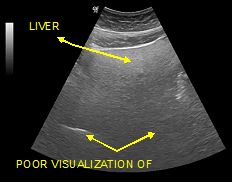
Figure 7 Poor visualization of the deeper parts of the organ and inability visualization of the diaphragm. (Grade III of fat filtration).
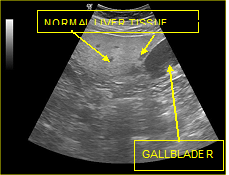
Figure 8 Focal fat infiltrations Islands of normal liver tissue within a sea of hepatic steatosis (presence of regions with normal echogenicity (islands) in the lipid-infiltrated parenchyma (sea).
Out of our total 1150 patients, 216 were afflicted with grade I fatty infiltration, 351 with grade II (Figure 9) and 19 with grade III (Figure 10). As of the 7 obese children the 2 were diagnosed with grade I fatty infiltration, whereas no ultrasonographic evidence were detected in the remaining 5 children.14 We can sonographically determine the grade of the diffuse hepatic steatosis based on the amount of lipids accumulated in the hepatocytes. Grading is used to communicate to the clinician the extent of the fatty changes in the liver.
Grading of fat infiltration with ultrasound (US Score)
Grade 0=Normal
Normal echogenicity of liver parenchyma, normal visualization of the diaphragm and intrahepatic blood vessels.
Grade I=Mild steatosis
Slightly increased echogenicity of liver parenchyma, normal visualization of the diaphragm and intrahepatic blood vessels.
Grade II=Moderate steatosis
Markedly increased echogenicity of liver parenchyma, slightly decreased visualization of the diaphragm and intrahepatic blood vessels.
Grade III=Severe steatosis
Severely increased echogenicity of liver parenchyma. No or severely decreased visualization of the diaphragm, intrahepatic blood vessels and posterior part of the right liver lobe.15,16
Ultrasonography (US) is often the first imaging modality used in clinical screening due to its widespread availability and low cost, compared to magnetic resonance-based or computed tomography techniques. Simple fatty infiltration of the liver is a condition which does not cause hepatic impairment to most people, however, steatohepatitis (fatty liver) may occasionally develop into cirrhosis (Figure 11),17 liver failure as well as hepatocellular cancer. The ultrasonographic examination is a simple test that is easy, fast and it’s the method of choice both for detecting non-alcoholic fatty liver disease as well as for clinically guiding patients with or without influenced hepatic biochemistry. The ultrasound method is very reliable in evaluating and staging fatty liver disease, thus helping the clinician in further therapeutic treatment. It has a high sensitivity of over 85% for grade I and 100% for II and III degree of fatty liver infiltration, but with low specificity probably due to the diseases’ coexistence with other pathological conditions such as fibrosis as a result of hepatic necrosis. Fat infiltration of the liver may be reversible if there is not an ongoing underlying process.18−22 In conclusion; ultrasound imaging is an important tool in diagnosing and staging NAFLD.23−32
Non-alcoholic fatty liver disease (NAFLD), fat infiltration, ultrasound.
None.
Author declares that there is no conflict of interest.

©2019 Glynou, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.