International Journal of
eISSN: 2574-8084


Research Article Volume 10 Issue 3
National Coalition of Independent Scholars, USA
Correspondence: Marco Ruggiero, MD, PhD, National Coalition of Independent Scholars. 125 Putney Rd Battleboro, VT 05301, USA
Received: July 28, 2023 | Published: August 9, 2023
Citation: Ruggiero M. Study of structural similarities between tubulin, TMC1, AND FTSZ proteins as they relate to mechanosensory transduction in the context of the orch or theory of consciousness. Int J Radiol Radiat Ther. 2023;10(3):65-72. DOI: 10.15406/ijrrt.2023.10.00358
Recent evidence obtained in murine atrial cardiomyocytes suggests that the behavior of tubulin, a constituent of microtubules, is influenced by audible sounds. In the present study, the sequence and the structure of murine and human tubulins were compared with those of human TMC1 (Transmembrane channel-like protein 1), a sound-sensitive protein that is responsible for forming the pore of mechanosensory transduction channels in the hair cells of the inner ear of vertebrates, and with those of bacterial FtsZ (Filamenting temperature-sensitive mutant Z), a bacterial protein homologous to tubulin. The results show that mouse and human alpha-tubulin are 100% identical, whereas sequence homology of human TMC1 and human tubulin alpha 1A chain is scarce, with only 17.31% identity. A higher degree of similarity was observed in the presence of aromatic amino acids as well as in the propensity to form alpha-helices. Based on these results, it is hypothesized that the response of tubulin to audible sounds is mediated by the external alpha-helices as it occurs in TMC1. The sequence homology of human TMC1 and FtsZ from Lactobacillus johnsonii, is moderate with 23.40% identity, however higher than that between TMC1 and tubulin. The overall presence of aromatic amino acids in FtsZ is scarce, but helix propensity is significant. These results are discussed in the context of Orch OR, a theory that postulates that consciousness emerges from quantum computations occurring in microtubules of neurons via information processing mediated by aromatic amino acids of tubulin.
Keywords: tubulin, TMC1, FtsZ, mechano-transduction, sound waves, Orch OR, microtubules
The Orch OR (Orchestrated Objective Reduction) theory of consciousness postulates that consciousness arises as a result of quantum computations occurring inside brain neurons, more precisely in microtubules that are structures of the cytoskeleton constituted by the protein tubulin.1 At the molecular level, microtubule information processing is hypothesized to be dependent on the presence of aromatic amino acids (tyrosine, tryptophan, and phenylalanine) in the helical structure of tubulin. Aromatic amino acids are characterized by ‘π’ orbital electron resonance clouds in indole and phenyl rings; these orbital clouds are made of electrons that are able to delocalize across a spatial region and would be responsible for quantum computations.1 Microtubules resonate in response to sound waves with frequencies ranging between 12 kHz and 30 MHz, that are frequencies in the range for diagnostic ultrasounds; it was hypothesized that these resonant frequencies could explain the effects of transcranial ultrasounds on mental states.2 Such an hypothesis received indirect confirmation by the observation that diagnostic ultrasounds at 12 MHz elicited changes in the cytoskeleton of human neuronal cells in culture; in ultrasound-treated cells, the number of elongations, as well as their maximum and mean lengths, increased significantly in comparison with control, untreated cells. In addition, ultrasounds induced cell differentiation affecting cell morphology, as well as the ability of neurons to form complex networks.3 Further studies demonstrated that diagnostic ultrasounds did not interfere with neuronal mitochondrial activity or protein folding.4
If microtubules respond to sound frequencies in the range for ultrasounds, recent observations seem to indicate that tubulin responds also to sounds in the range for audible since it was demonstrated that audible sound stimuli changed the average fractal dimension and lacunarity of alpha-tubulin in the cultured murine cardiac muscle cell line HL1.5 Therefore, it may be hypothesized that audible sounds, just as ultrasounds, may affect mental states by acting on tubulin in the context of the microtubules of neurons. In order to prove this hypothesis, the sequence and structure of tubulin and of its bacterial counterpart, FtsZ (Filamenting temperature-sensitive mutant Z), were compared with those of TMC1 (Transmembrane channel-like protein 1) a sound-sensitive protein that is responsible for forming the pore of mechanosensory transduction channels in the hair cells of the inner ear of vertebrates.6
The rationale for including FtsZ lays in the observation that microbes show the attribute of intelligent behavior7 and consciousness. Such a microbial consciousness derives from quantum-mediated computations in cytoskeletal structures, that is in a manner superimposable to that described by Orch OR.8 Since there exists a brain microbiome9-12 where microbes live in symbiosis with neurons and glial cells, it is conceivable that microbes contribute to human consciousness in many ways that comprise participation in Orch OR.13
Study of sequences and structures of alpha-tubulins and TMC1
Sequences and structures of proteins were studied using the database UniProt and, more specifically, the function designated "align".14
Since the results on audible sounds and alpha tubulin were obtained in murine cells,5 as a first step it was necessary to assess whether murine and human alpha-tubulin shared homologies. Figure 1, shows that murine and human alpha-tubulin are 100% identical.

Figure 1 Results of alignment of murine tubulin alpha 1A chain (upper line) and human tubulin alpha 1A chain. Alignment of murine and human tubulin alpha-1B chain gave identical results.
Since such an identity extends to aromatic amino acids, it may be deduced that the information processing ability of murine tubulin is identical to that of human tubulin and, therefore, that the results described by Dal Lin et al.5 may be extrapolated to humans.
The following step was to establish whether human tubulin shares homologies with TMC1, a protein that is known to transduce audible sounds.6 Figure 2, shows that the sequence homology of human TMC1 and human tubulin alpha 1A chain is scarce, with only 17.31% identity.
A higher degree of similarity can be observed in the presence of aromatic amino acids (Figure 3) as well as in the propensity to form alpha-helices (Figure 4) that are the amino acids and the secondary protein structures where, according to Orch OR,1 information processing occurs.

Figure 3 Results of alignment of human tubulin alpha 1A chain (upper line) and TMC1. Aromatic amino acids Tyrosine, Y; Tryptophan, W; and Phenylalanine, F are highlighted. Histidine (H) although not comprised in Orch OR as reported by Hameroff and Penrose (2014), is here highlighted since it can be considered aromatic (Anjana et al. 2012).

Figure 4 Results of alignment of human tubulin alpha 1A chain (upper line) and TMC1. The amino acids that occur more frequently in alpha-helices are highlighted (Pace and Scholtz 1998).
It should be noticed that both proteins show a secondary structure that is typical for proteins participating in the formation of supramolecular cylindrical structures with an inner cavity (microtubules for tubulin; pores for TMC1). Fig. 5, shows the comparison between the secondary structures of tubulin and TMC1; the alpha-helices positioned external to the beta-sheets are well visible.
Based on these results, it may be hypothesized that the response of tubulin to audible sounds, as reported by Dal Lin et al.,5 is mediated by the external alpha-helices as it occurs in TMC1; consequently, the changes in the spatial morphology of tubulin has to be associated with changes of information processing via aromatic amino acids and, therefore, with changes of consciousness, mental states or brain activity as it occurs with exposure to ultrasounds.2,15
Study of sequences and structures of human TMC1 and bacterial FtsZ
It is well assessed that bacterial FtsZ is related to tubulin of eukaryotes;16 actually, this was the first observation that bacteria have homologs of proteins typical of the eukaryotic cytoskeleton. Since bacteria emit and respond to audible sounds17 and their "intelligent" behavior is influenced by sound,18 the sequence and the structure of FtsZ were compared with those of human TMC1. The rationale for this comparison lays in the consideration that if FtsZ, being a homolog of tubulin, may be responsible for microbial consciousness, and if sound affects bacterial behavior, and hence, consciousness, it is possible that FtsZ has sequences, or structures, able to be modified by sound.
The sequence homology of human TMC1 and cell division protein FtsZ from Lactobacillus johnsonii, a probiotic bacterium19 is moderate with 23.40% identity, however higher than that obtained by comparing human TMC1 and tubulin (Figure 5). Helix propensity is significant in the region comprising amino acids 18-195 of human TMC1 (Figure 6).
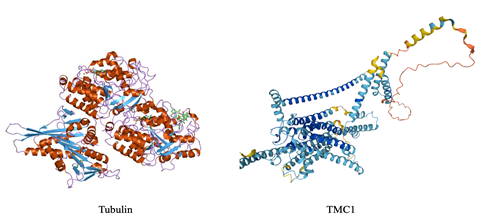
Figure 5 Secondary structure of human tubulin (cartoon representation of the molecular structure of protein registered with 1ia0 code by Jawahar Swaminathan and MSD staff, https://www.ebi.ac.uk/), and human TMC1 (Ruggiero, https://www.uniprot.org/uniprotkb/Q8TDI8/entry).

Figure 6 Results of alignment of human TMC1 (upper line) and bacterial FtsZ. The amino acids that occur more frequently in alpha-helices are highlighted (Pace and Scholtz 1998).
In the region comprising amino acids 18-515 of human TMC1, five aromatic amino acid alignments were observed; however, the overall presence of aromatic amino acids in FtsZ is scarce (Figure 7). If information processing in cytoskeletal structures depends on aromatic amino acids (Hameroff and Penrose 2014), then the computational power of prokaryotes, or at least that of this Lactobacillus, appears to be lower than that of eukaryotes.

Figure 7 Results of alignment of human TMC1 (upper line) and bacterial FtsZ. Aromatic amino acids Tyrosine, Y; Tryptophan, W; and Phenylalanine, F are highlighted. Histidine (H) although not comprised in Orch OR as reported by Hameroff and Penrose (2014), is here highlighted since it can be considered aromatic (Anjana et al. 2012).
Figure 8, shows the comparison between the structures of FtsZ and TMC1; the alpha-helices positioned external to the beta-sheets are well visible.
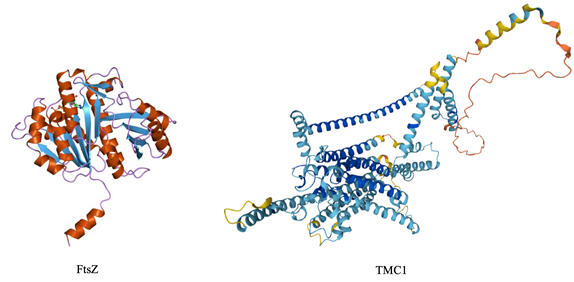
Figure 8 Secondary structure of bacterial FtsZ (cartoon representation of the molecular structure of protein registered with 1fsz code by Jawahar Swaminathan and MSD staff, https://www.ebi.ac.uk/) and human TMC1 (Ruggiero, https://www.uniprot.org/uniprotkb/Q8TDI8/entry).
Based on these results, it may be hypothesized that bacterial FtsZ, in analogy with tubulin, responds to audible stimuli, possibly through mechanical vibration of the external helices.
Study of sound analysis used to evoke tubulin responses
In the study of Dal Lin et al.5 the murine atrial cardiomyocyte cell line HL1 was stimulated with a series of audible sounds for 20 min and tubulin behavior was studied by immunofluorescence. Among the different stimuli, a meditative phoneme of two syllables (mantra) induced the greatest increase in average fractal dimension and the greatest decrease of lacunarity (Figure 9).

Figure 9 (A) Starting condition, description in the text [of Dal Lin et al. (2021)]. (B) Alpha-tubulin staining after the different 20-min sounds stimulation with a graph below representing ƒ(α) vs. α (the typical pattern for multifractals) and the results of the multifractal analysis reporting the average fractal dimension (D) and lacunarity (L). Figure and legend reproduced from Figure 1 of Dal Lin et al. (2021) under the terms and conditions of the Creative Commons Attribution (CC BY) license https://creativecommons.org/licenses/by/4.0/
Figure 10, shows comparison of sound analysis of the phoneme of two syllables (mantra, left panel) used by Dal Lin et al.5 with the hexameter chant (Nam-Myoho-Renge-Kyo; right panel) that has been studied for its association with psychological resources,20,21 and its effects on electrolytes.22,23
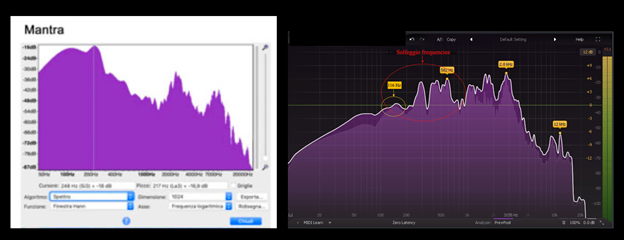
Figure 10 Left panel: sound analysis reproduced from Figure 3 of Dal Lin et al. (2021) under the terms and conditions of the Creative Commons Attribution (CC BY) license https://creativecommons.org/licenses/by/4.0/. Right panel: sound analysis using a FabFilter Pro-Q3 high quality equalizer. Nam-Myoho-Renge-Kyo chanting at Taisekiji Temple in Japan (https://www.youtube.com/watch?v=p2F488BV3oA). The frequency at 116 Hz is indicated by a yellow circle. The area of the solfeggio frequencies is indicated by a red circle.
It has been known since antiquity that sounds affect human mental states and physiology; several studies have described the neurophysiological responses to different types of sounds ranging from recitation of the Odissey24 to religious chanting.25 However, just as it happens for murine cardiomyocytes in vitro,5 different types of sounds elicit different responses. For example, recitation of Homer's Odyssey translated in German, a language that maintains the original hexametric pace of the verse that is six feet, or rhythmic units, per line, leads to synchronization of heart rate and respiration24 up to the point that it is being proposed in the context of the Anthroposophical Therapeutic Speech; this is an approach aiming at the optimization of physiological processes through synchronization of heart rate and respiration, and rebalancing of the sympathovagal equilibrium.26 On the other hand, Buddhist religious chanting induces functional changes in the brain that are not ascribable to changes of respiratory or cardiac activity, nor to the implicit processing of language; these changes appear to be different from those induced by meditation or prayer.25 It is worth noticing that the religious/spiritual dimension has a significant impact on the state of mind.27,28 and such a dimension, or the absence of it, might explain the differences observed by Gao et al.25
The spiritual dimension in reciting an hexameter in the context of religious experience pertains to the chanting of Nam-Myoho-Renge-Kyo, a practice that originated in Japan in 1253 and is now carried out on a daily basis by millions of followers of Nichiren Daishonin's Buddhism all over the world. The word Nam derives from the Sanskrit word namas and means "devotion". Myoho-Renge-Kyo is the title of the Lotus Sutra in Japanese20,21,29 A simplified translation in English could be "Devotion to the Mystic Law of the Lotus Sutra".
The psychological aspects associated with the assiduous practice of Nichiren Daishonin's Buddhism have been published in a few studies. A study published in 201820 compared the psychological resources of 60 practitioners of Nichiren Daishonin's Buddhism, the most widespread branch of Buddhism in Italy, with those of non-practicing Roman Catholic Church believers and those of Atheists. It was observed that those who assiduously chanted Nam-Myoho-Renge-Kyo and practiced within a community of believers had higher optimism than both Catholics and Atheists. Practitioners of Nichiren Daishonin's Buddhism also showed higher self-efficacy and self-esteem than Catholics and higher perceived social support than Atheists. As far as global personality factors were concerned, Buddhists were more extraverted than the other groups, and they appeared less tough-minded than Catholics. Significant differences were also observed in primary personality factors. Having observed no differences between Catholics and Atheists, the Authors concluded that what is determinant for the greater psychological resources of Buddhists, is the religious practice, that is the assiduous chanting of Nam-Myoho-Renge-Kyo and practice within a community of believers. Another study published in 2019,21 was conducted on 391 practitioners of Nichiren Daishonin's Buddhism in Italy; the results were consistent with those of the previous study. Buddhist subjects made use of adaptive coping strategies, had a predominant internal locus of control, and showed a low psychopathological profile when compared with the normative standard scores.
As far as the actual practice of Nichiren Daishonin's Buddhism is concerned, believers recite aloud (not silently) Nam-Myoho-Renge-Kyo for several minutes every day while performing a liturgy that is called Gongyo, a word originated in ancient China that can be translated as "assiduous practice". During chanting, believers keep their eyes open focusing on the object of worship as per the liturgy of Nichiren Shoshu. They typically repeat Nam-Myoho-Renge-Kyo approximately 15-20 times every breath with a frequency of approximately 3-4 respiratory cycles per minute with rapid inspirations and prolonged exhalations during chanting. Chanting Nam-Myoho-Renge-Kyo with a breathing pattern characterized by rapid inspirations and prolonged exhalations may have significant consequences on brain physiology at the light of the results reported by Dal Lin et al.5 and in the context of Orch OR. For example, it was demonstrated that the thickness and the water content of the brain cortex and the meninges vary with inspiration and exhalation; during exhalation, the thickness and the water content are greater.31-33
Figure 11, reproduced below from Ruggiero et al.,31 shows the changes of thickness and water content of brain cortex and meninges with inspiration and exhalation
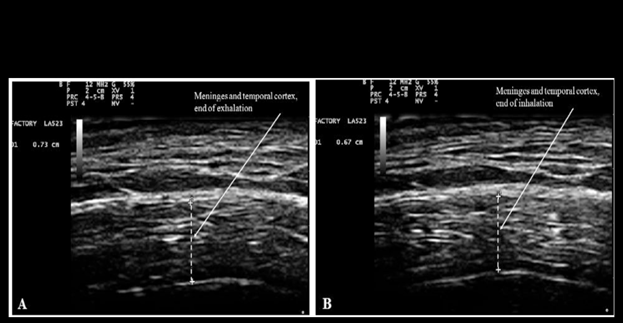
Figure 11 Measurement of combined thickness of the meninges and the cortex of the temporal lobe at the end of forced voluntary exhalation (A: 7.3 mm), and inhalation (B: 6.7 mm). Reproduced from Figure 5 of Ruggiero et al.46 under the terms and conditions of the Creative Commons Attribution 4.0 International Public License (CC-BY-4.0) license https://creativecommons.org/licenses/by/4.0/.
Therefore, during prolonged exhalation, as it occurs in Nam-Myoho-Renge-Kyo chanting, the sound waves generated by chanting take a longer time to travel through areas of the brain that have a greater content in water in comparison with the same areas during inspiration. Since sound waves are known to modulate the expression of a number of genes in eukaryotic cells,34 and are also able to alleviate chemical stress in microbial cells,18 it may be hypothesized that the greater thickness and content of water give more time to the sound waves to exert their beneficial effects35 on the human cells of the brain as well as on the microbial cells of the brain microbiota9-12 In addition, chanting is associated with increase in blood flow in the prefrontal cortex,36 and this would also contribute to the increase in water content, thus further favoring the effects of the sound waves on the human and microbial cells of the brain. It may be worth considering in this context that microbial cells are particularly represented in the prefrontal cortex as well as in other critical areas of the human brain such as the hippocampus and the substantia nigra12 where they may play roles not less significant than those played by neurons or glial cells. For example, it was proposed that the properties of the brain microbiota could be integrated in the Orch OR theory of consciousness, thus expanding its explanatory and predictive powers.13 It should also be considered that microbes exhibit the attributes of consciousness and the molecular mechanisms responsible for microbial consciousness are the same that are at the basis of human consciousness.8 Therefore, it is not implausible to hypothesize that recitation of Nam-Myoho-Renge-Kyo acts not only on human cells but also on the cells of the human microbiota whether they are located in the brain or elsewhere. In microbial cells such an action may be mediated by FtsZ thanks to its homology with tubulin and its structural similarity with TMC1 as described above. It is interesting to notice that such a concept is not in contradiction with Buddhism. In a writing of Nichiren Daishonin of 1255 is written Life at each moment encompasses both body and spirit and both self and environment of all sentient beings in every condition of life as well as all insentient beings in every condition of life, as well as insentient beings - plants, sky, and earth, on down to the most minute particles of dust. Life at each moment permeates the universe and is revealed in all phenomena.37 These words are significantly reminiscent of the words written 758 years later by Nobel Laureate Sir Roger Penrose and Professor Stuart Hameroff ... there is a connection between the brain's biomolecular processes and the basic structure of the universe. ... We conclude that consciousness plays an intrinsic role in the universe.1
The role of sound in explaining some of the effects observed when chanting is further supported by the observation that vocalization of Nam-Myoho-Renge-Kyo is associated with a sound at 116 Hz22,38,39 as well as with the so-called Solfeggio frequencies ((Figure 10). The low-frequency sound at 116 Hz is perceived by the Buddhist practitioner as a continuous humming that accompanies chanting. The frequency at 116 Hz is particularly interesting because of its interactions with water that contains electrolytes. These interactions are so effective that this frequency has been proposed as a tool for water purification and desalinization.22,23 It could be therefore hypothesized that changes in electrolyte composition in the aqueous environment of the brain, with particular reference to decrease of chloride ions, as result of interaction with a 116 Hz sound,23 occur during chanting of Nam-Myoho-Renge-Kyo with consequent changes of cell depolarization patterns and synaptic transmission. The Solfeggio frequencies associated with chanting, on the other hand, may be responsible for a series of biological effects that may help explaining the increase in psychological resources described by Giannini et al.,21 and Bragazzi et al.10 It has been demonstrated that these frequencies have a positive impact on the human endocrine and autonomous nervous system40 and improve survival of human brain astrocytes in vitro.41 It appears that the positive effects of these frequencies, however, are not limited to humans since it has been demonstrated that they decrease anxiety in rats41,42 and reverse cognitive and endocrine deficits in zebra fish (Dos Santos et al.43), thus indirectly confirming the hypothesis enunciated above that recitation of Nam-Myoho-Renge-Kyo acts not only on human cells.
It should also be considered that the increase in brain activity occurring during chanting, as it is reflected by the increase in psychological resources,20,21 is associated with changes of the brain electromagnetic field, a field that can be measured by magnetoencephalography44 and is presumed to be able to transmit information.45 In fact, the increase in blood flow in the prefrontal cortex associated with the increase of synaptic activity and with the changes in electrolyte composition due to the sound waves, might generate an unique pattern of change of the brain electromagnetic field. The concept of an electromagnetic field emanating from the forehead is well known in the Buddhist tradition; in the Lotus Sutra is described how Shakyamuni Buddha emitted a ray of light from his forehead. Since it has been recently demonstrated that humans have indeed the ability to perceive magnetic fields as weak as the earth magnetic field via a quantum mechanical magnetoreception mechanism46,47 could it be that the imaginative description of the Lotus Sutra actually reflected an electromagnetic event originating from the brain? And, could it be that these changes of the brain electromagnetic field allow connection with the intrinsic consciousness of the universe as proposed by Penrose and Hameroff?1 The hypothesis is all the more intriguing if we consider that although sound waves cannot directly interact with electromagnetic waves, when they both share a common medium, and that medium has electrical properties that vary with mechanical strain, as it is exactly what happens in the brain as also demonstrated in Figure 11 and in Ruggiero et al.,48 the two undulatory phenomena actually interact. As a matter of fact, opto-acoustical devices for medical imaging49 or the technique called "chirp" for enhancing radar performance, utilize the interaction of sound and electromagnetic waves. From a theoretical standpoint, microtubules could be considered a sort of resonant cavity filled with a piezoelectric material, e.g. proteins50,51 Resonant standing waves, either electromagnetic or acoustical, will then produce fixed patterns of electromagnetic or acoustic properties in microtubules.52 In other words, according to this hypothesis, microtubules would work as Fabry-Pérot interferometers able to detect and interpret, in the context of Orch OR, the interaction of sound and electromagnetic waves. It is therefore plausible that the electromagnetic and sound waves generated during recitation of Nam-Myoho-Renge-Kyo interact with each other in the context of microtubules and are interpreted by the information processing machinery of the microtubules, ultimately contributing to changes in the level of consciousness associated with the increase of psychological resources observed in the Italian Buddhist practitioners20,21 The interaction of the sound waves generated while chanting with the electromagnetic waves generated by brain activity therefore leads to an electromagnetic/vibrational signature that is unique for each type of chanting or recitation. In other words, even assuming that the chanting of different mantras such as OM28 or Saa Taa Naa Maa,53 or the recitation of Greek Hexametric verses,24 might activate the same areas of the brain, nevertheless since the sound generated by the vocalization of each mantra/recitation is different, the signature of each electromagnetic/vibrational interaction and its effects on human, or microbial, cells are different and not interchangeable. For example, recitation of Nam-Myoho-Renge-Kyo produces results different from those deriving from recitation of other mantras or prayers or recitation of the Odyssey. To this point, the author wishes to clarify that the present study has no pretension of comparing different approaches to meditation nor different religions. More importantly, it has no pretension of trying to reduce a highly regarded religious practice such as Nichiren Daishonin's Buddhism to neurophysiological mechanisms operating in the brain or elsewhere. The religious and spiritual experience of practicing Buddhism and chanting Nam-Myoho-Renge-Kyo goes well beyond brain activity, neurotransmitters or quantum phenomena of consciousness; it encompasses a mystic dimension that, although not in contradiction with science, can't be explained solely by science. Nevertheless, Nichiren Daishonin's Buddhism states that Buddhism is reason54 and Nothing is more certain than actual proof55; the author believes that, despite its limitations, this study could be considered a contribution to the "actual proof".
The analysis of the sequences and structures of tubulin, FtsZ, and TMC1 suggests that these proteins are able to transduce sound waves in a medium with internal forces of elastic or viscous nature. In other words, they are able to intercept oscillations in pressure, displacement of particles and their velocity, and the resulting stress exerted on their molecular structure. These features explain the changes in tubulin behavior observed by Dal Lin et al.5
Therefore, the following sequence of events may be suggested:
As shown in Fig. 12, the repetitive generation and exposure to sound leads to recursive functions that are generated, on one side, by the sensitivity of tubulin to sound, and, on the other, by the ability of microtubules to work as interferometers, interpret the interactions of electromagnetic sound waves, and modify accordingly axonal firing leading to increased brain activity.
The author wishes to thank Filippo Ruggiero for support in sound analysis; Dr. Akira Hiratsuka for his support in the interpretation of the 116 Hz frequency. The author acknowledges the great work of Dr. Aldo Ruggiero, MD (1923-2006), pioneer of radiology in Prato, Italy, founder of the Studio Radiologico Ruggiero, source of boundless inspiration for this and many other scientific articles.
The author contributed solely to the work.
The author declares that he has no conflicts of interest concerning the topics described in this article.
Not applicable. This article does not report experiments performed on humans or animals. The results depicted in Fig. 11 were previously published in Ruggiero et al.31 These results are here illustrated, and their significance elaborated, in the context of the object of this article.
Not applicable.
The author did not receive any funding for this study.

©2023 Ruggiero. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.