International Journal of
eISSN: 2574-8084


Case Report Volume 4 Issue 2
1Department of Radiation Oncology, University of Nebraska Medical Center, USA
2Department of Neurosurgery, University of Nebraska Medical Center, USA
Correspondence: Weining K Zhen, Department of Radiation Oncology, University of Nebraska, 987521 Nebraska Medical Center, Omaha, NE 68198, USA, Tel (402) 552-3844, Fax (402) 552-3013
Received: August 19, 2017 | Published: August 29, 2017
Citation: Zhen WK, Denniston KA, Li S, et al. Spatial displacement of a single brain metastasis after corticosteroid treatment and its impact on stereotactic radio surgery planning and delivery. Int J Radiol Radiat Ther. 2017;4(2):343-347. DOI: 10.15406/ijrrt.2017.04.00091
brain metastasis, intra-cranial, treatment, melanoma, neurosurgical
SRS, stereotactic radiosurgery; CT, computed tomography; MRI, magnetic resonance imaging; GTV, gross tumor volume
Stereotactic radio surgery (SRS) has been widely used for brain metastasis.1‒4 The delivery of a high dose of ionizing radiation that conforms to the shape of the lesion mandates an overall accuracy of approximately 1mm.5 SRS is image-based treatment. All salient anatomical features of the SRS patient, both normal and abnormal, are defined with computed tomography (CT), magnetic resonance imaging (MRI), and/or other applicable imaging modalities. Both high three dimensional spatial accuracy and tissue-contrast definition are very important imaging features if one is to utilize SRS to its fullest positional accuracy. Accurate, image-based target delineation requires the pre-condition or assumption that there is no change in either target shape or anatomic location between the time of imaging and the time of treatment planning and delivery. Intra-cranial targets are generally considered fixed and motionless. Therefore, it is unusual to see significant migration of an intra-cranial target. Herein, we report the case of a patient with significant brain tumor displacement secondary to the effect of pre-SRS corticosteroids, which could have potentially resulted in a significant treatment miss-delivery.
The patient is a 64year-old female who in 2004 was diagnosed with melanoma involving the skin of the right shoulder. This was treated with a wide local excision and sentinel lymph node biopsy. Pathology showed melanoma with a Breslow depth of 4.0mm with negative surgical margins. One sentinel lymph node was negative for metastasis. No adjuvant therapy was given. She did well until July 2012, when she developed progressive left upper extremity weakness and headaches. Brain MRI on August 5, 2012 showed a 2.8x2.5x2.8cm round mass, centered within the right pre-central gyrus, with diffuse, homogeneous, gadolinium contrast enhancement and significant surrounding vasogenic edema (Figure 1A) (Figure 1B). CT scan of the chest and abdomen demonstrated a 5.2x3.9cm mass in the right lower lobe of the lung along with two left lower lobe pulmonary nodules. She was started on dexamethasone (4mg q 6hours) and her headaches quickly resolved. The left upper extremity weakness also greatly improved.
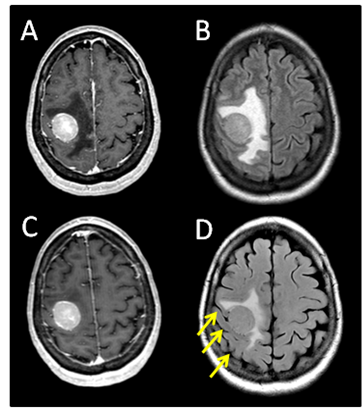
Figure 1 (A) Pre-steroid T1 MRI with contrast. (B) Pre-steroid T2 MRI with FLAIR. (C) Post-steroid T1 MRI with contrast. (D) Post-steroid T2 MRI with FLAIR. Arrows depict reappearing sulci after steroid treatment.
The patient was initially evaluated by the neurosurgical team, but because of the eloquent location of her brain metastasis, in the pre-central gyrus, craniotomy and tumor resection were not recommended. She was referred to radiation oncology on August 7, 2012 for consideration of palliative radiation therapy for her single brain metastasis. Physical examination at the time of consultation showed only minimal residual weakness in her left hand. Her ECOG performance score was 0. In light of her single lesion and presumed melanoma histology, SRS was deemed to be appropriate and was recommended. On August 14, 2011, the patient underwent a CT-guided fine needle aspiration of the right lung mass. Pathology confirmed metastatic melanoma.
Radiation treatment planning and delivery
The patient returned for simulation in preparation for frameless SRS on August 17, 2012, 12days after her initial brain MRI. CT simulation was performed using 2.4mm slice thickness. The Brain LAB Novalis Radio surgery System (iPlan RT V4.1, BrainLAB, Heimstetten, Germany) was used for the treatment planning and delivery. The original (pre-steroid) MRI from August 5, 2012 was registered and fused to the CT images for target delineation. The gross tumor volume (GTV) was contoured based on the isotropic 1mm thickness 3D MRI using a gadolinium enhanced T1 sequence (Figure 2A). However, when the GTV was superimposed on the CT images, it became clear that there was a significant mismatch. The hyperdense metastatic lesion was quite easily seen on the simulation CT images, even without IV contrast (Figure 2B). This prompted the decision to abort SRS. A repeat MRI was obtained on August 21, 2012 using identical imaging parameters. The new MRI showed stable enhancing right posterior cortical mass, but with improved surrounding vasogenic edema (Figure 1C) (Figure 1D). A second treatment plan was constructed based on the new MRI. The patient underwent SRS treatment on August 22, 2012. The final planning target volume (PTV) was 11.69cm3. Because of the relatively large volume (>10cm3) and eloquent location of the tumor, it was decided to prescribe 16Gy to the 90% isodose volume. Prior to dose delivery, target-localization was performed using the Brain LAB image-guided positioning system (ExacTrac V5.5.2). The treatment was delivered via 6 non-coplanar dynamic arcs, without difficulties. The patient tolerated SRS well.
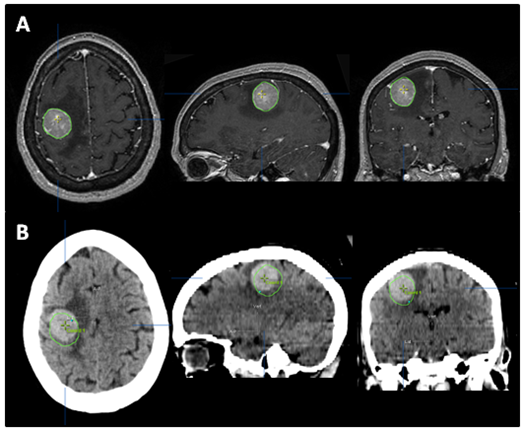
Figure 2 The tumor was contoured (green line) on pre-steroid MRI (A) and superimposed on CT simulation images (B). The tumor was identified as a well-defined, hyperdense mass on planning CT, even without IV contrast. Interval tumor migration has clearly occurred during 12 days between the acquisitions of these two scans.
Treatment outcomes
After SRS, the patient continued to have very mild, stable, left hand weakness. Her first follow up brain MRI on September 25, 2012 (4.5 weeks after SRS) showed good initial response (Figure 3B). Approximately 4weeks after SRS, however, she became more symptomatic from the intra-thoracic disease and subsequently received palliative radiation to the right hilar mass with a total dose of 30Gy in 10 fractions. She had excellent response to the chest radiation, both radiologically and symptomatically. She was then started on systemic therapy with vemurafenib.
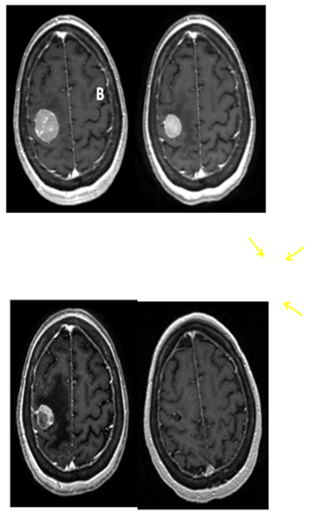
Figure 3 Serial T1 with contrast MRI scans. (A) Pre-SRS. (B) 5 weeks post SRS. (C) 9 weeks post SRS showing the tumor to be largely necrotic, though with increased edema (arrows). (D) 5 months post SRS showing near complete resolution of contrast enhancement and edema.
The patient did well until October 23, 2012 (2months after her SRS) when she developed 2 episodes of seizure activity involving her left arm and hand without loss of consciousness. A brain MRI demonstrated increased vasogenic edema around the previously irradiated, right posterior frontal lobe lesion. The tumor had become largely necrotic (Figure 3C). There was no interval development of new metastatic foci. She was started on levetiracetam and was also given dexamethasone for the treatment of possible radiation related brain edema, which was eventually, successfully weaned off without problems. She has subsequently remained seizure free. Neurologically, she was doing well with mild residual impairment of fine motor function in her left fingers, but free of other neurologic deficits. Her overall functional status had also remained stable at ECOG 1. She was last seen in radiation oncology clinic on January 25, 2013 (5months after SRS) and was noted to be doing well. Brain MRI showed near complete resolution of the contrast enhancement and vasogenic edema of the brain lesion Figure 3D.
Brain metastases are a common complication of systemic cancer and the most common intracranial tumor in adults. The incidence of brain metastasis is rising with the increase in survival of cancer patients. Melanoma is the third most common underlying diagnosis in patients with cerebral metastases, only behind lung and breast cancers.6 Autopsy data have shown that up to 75% of patients who died from metastatic melanoma had brain metastases at the time of death.7,8 Metastatic melanoma in the brain has a poor prognosis and may significantly impair quality of life. Symptomatic brain metastases represent the initial site of disseminated disease in about 20% of patients, but may occur at any time during the course of the disease.9 The number of cerebral metastases is a significant prognostic factor with better prognosis seen in single or oligometastatic disease (2-3 cerebral metastases).10 SRS has been shown to be effective and even advantageous with or without whole brain irradiation for solitary metastasis, particularly for metastatic melanoma. A series of the University of Pittsburgh’s experience treating 333 consecutive patients with 1,570 metastatic melanoma lesions with Gamma Knife SRS showed a local control rate of 73%, with an actuarial overall survival rate of 70% at 3months, 47% at 6months, 25% at 12months, and 10% at 24months.11
SRS is image-based treatment. All salient anatomic features of the SRS patient, both normal and abnormal, are commonly defined with CT and/or MRI. Both high three dimensional spatial accuracy and tissue-contrast definition are critical to the optimal definition of target(s) and critical structures for SRS. Imaging, whether CT or MRI, is crucial for localizing target boundaries as well as generating target coordinates for accurate treatment beam localization. Precise SRS treatment planning and delivery requires as a prerequisite that the target position is unchanged between image acquisition and treatment planning and delivery. In general, an intracranial target is not considered a moving target. Therefore, with rigid image registration and fusion, the MRI defined target and normal structures should accurately represent their relative physical position in space. Re-imaging of the brain is often done to evaluate for possible tumor progression, however, target migration between the time of imaging and treatment planning and delivery is typically not a concern. The present case illustrates a potential pitfall in the commonly made assumption that the location of the intra-cranial target is fixed in relation to the cranium and surrounding structures. In this study, the tumor position shifted within 12days, as shown in Figures 2 & 4. This resulted in a significant GTV mismatch of 3.312cc (28.3%) and a shift of the isocenter by 3.4 mm posteriorly, 0.3mm laterally and 2.5mm inferiorly (Figure 4). As demonstrated in Figure 5, if the pre-steroid MRI had been used for target delineation and planning, a significant portion of the GTV would not have been covered by the prescription dose (90% isodose line). The DVH confirmed that only 83% of GTV would have received the prescribed dose (Figure 6). Conversely, this could also have led to a significant overdose to the normal brain, in this case, the post central gyrus (Figure 2B) (Figure 2C). Quantitatively, 0.47cm3 more normal brain would have received the prescription dose of 16Gy, an increase of 17% compared to the treatment plan that the patient had actually received (3.24cm3 vs. 2.77cm3, respectively). Evidently, dexamethasone significantly decreased the peritumoral vasogenic edema, allowing the tumor to shift anteriorly and decompressing the post-central gyrus. If undetected, this would have represented the worst scenario for SRS, manifesting both inadequate tumor coverage, and extremely high normal tissue dose.
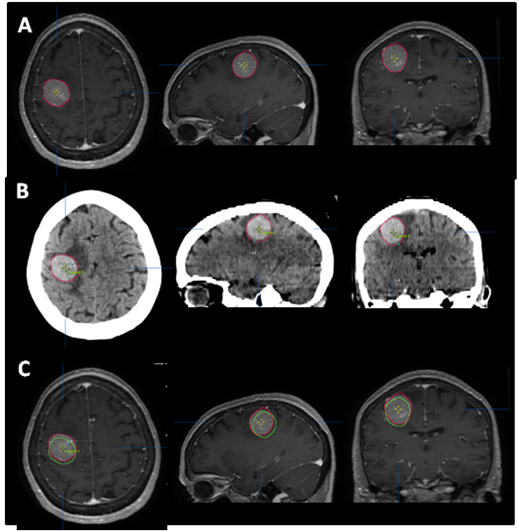
Figure 4 Tumor contour. The red line (narrow arrow) depicts the target contoured on the post-steroid MRI (A), which perfectly superimposed the tumor seen on the CT (B). The green line (broad arrow) represents the tumor contour based on the pre-steroid MRI. (C) depicts the superimposition of the two contours, and illustrates the magnitude of tumor displacement.
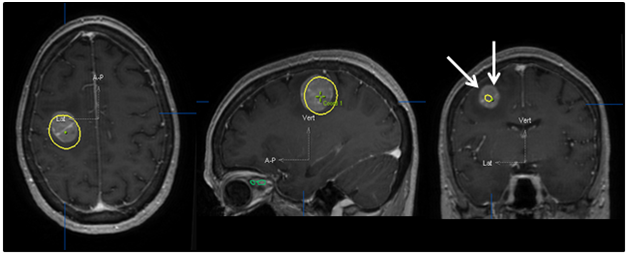
Figure 5 Original treatment plan superimposed on post-steroid MRI. The yellow line shows the 90% isodose line that was used for the dose prescription. This clearly demonstrates grossly inadequate dose coverage as shown by the arrows if the plan had been constructed using the pre-steroid MRI imaging.
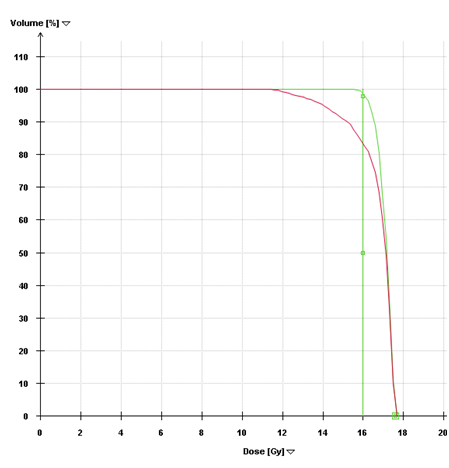
Figure 6 Dose-Volume Histograms. The green line (narrow arrow) is the DVH for the pre-steroid MRI target volume and demonstrates that 98% of this volume was covered by the prescribed dose in the initial plan. The red line (broad arrow) is the DVH for the post-steroid target volume, using the same treatment plan. Only 83% of the target volume would have received the prescription dose if the initial plan had been used.
Tumor migration in this patient only became evident because of the hemorrhagic nature of melanoma on the treatment planning CT. Hyperdensity on non-contrast CT is commonly seen with metastases from melanoma, renal cell carcinoma and thyroid carcinoma. If this were not the case, our patient could have received compromised SRS as the majority of brain metastases would be poorly defined or un-identifiable on CT scan, even with IV contrast. Since CT simulation scans are typically obtained without IV contrast, planning MRI is critical for target delineation and identification of normal structures.
Vasogenic edema is a prominent feature of brain metastases, and is the result of plasma leakage into the brain parenchyma through dysfunctional cerebral capillaries. It is often associated with headaches, neurologic dysfunction and impaired quality of life. Corticosteroids are the mainstay of treatment for vasogenic edema and dexamethasone is the most commonly used corticosteroid for this purpose. In this setting, corticosteroids work by reducing the expression of the edema-producing factor VEGF.12,13 The edema-reducing effect of corticosteroids can be rapid and reductions in capillary permeability can be evident within an hour after a single dose of dexamethasone.14 A study by Hatam et al.15 demonstrated a marked linear decrease within 2weeks in cerebral metastasis induced edema with the treatment of dexamethasone.6 A quantitative MR analysis of glucocorticoid effects on metastatic peritumoral edema showed 20% reduction in edema after 24hours, 35% after 3days, and 64% after 7days of glucocorticoid treatment11. However, the correlation between the reduction of edema associated with cerebral metastases and the actual reduction of the mass effect is more complicated and has not been reported. Nonetheless, if there is significant vasogenic edema that has been treated with high dose corticosteroids, re-imaging prior to treatment planning is necessary to avoid a marginal miss should SRS be performed. It is noteworthy that this patient did develop symptomatic brain edema about 2months after SRS (Figure 3C). The incidence of progression or worsening of symptomatic cerebral edema after SRS has been reported in 4–6% of patients within 1-2weeks of treatment. Seizures within 1-2days in 2–6%, and delayed radiation necrosis in 2–11% have also been reported.16,17 The risk of post SRS edema increases with several factors such as prior treatment, larger treatment volumes (both larger lesions and larger numbers of lesions), and higher doses delivered.
This report highlights the risk of brain tumor displacement associated with the use of corticosteroids for the management of acute vasogenic edema. We conclude that, if there is significant peritumoral edema present, which has been treated with high dose corticosteroids, SRS treatment planning MRI should be performed at the time or as close to the time of treatment planning and delivery as possible.
The authors report no conflict of interest concerning the materials or methods used in this study or the findings specified in this paper.
None.
Author declares that there is no conflict of interest.

©2017 Zhen, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.