International Journal of
eISSN: 2574-8084


Research Article Volume 7 Issue 1
Philipps-University Marburg, Klinik für Strahlentherapie and Radioonkologie, Germany
Correspondence: Christoph Dumke, Klinik für Strahlentherapie, University Hospital of Giessen and Marburg, Germany, Tel 064215864690
Received: January 17, 2020 | Published: February 20, 2020
Citation: Dumke C, Mortasawi F, Thiemer M, et al. PSA outcome after metastasis directed therapy (MDT) of oligometastatic prostate cancer patients diagnosed by 68Ga-PSMA-PET/CT. Int J Radiol Radiat Ther. 2020;7(1):24-32. DOI: 10.15406/ijrrt.2020.07.00259
Background: Metastasis Directed Therapy (MDT) of oligometastatic prostate cancer (OPCa) is an emerging treatment option which may delay systemic therapy and its side effects. Identifying OPCa patients suitable for local treatment by imaging modalities with a high sensitivity and specificity is crucial. Therefore, we analyzed PSA outcome after MDT of OPCa patients diagnosed by 68Ga-PSMA PET/CT retrospectively.
Methods: Overall 20 OPCa patients with ≤3 metastases in 68 Ga-PSMA PET/CT treated with radiotherapy (RT) doses >50 Gy (EQD2, α/β=2) were identified. Biochemical progression free survival (bPFS) was calculated with Kaplan Meier method. After stratifying patients by clinical and pathological parameters, differences in bPFS were compared using log rank tests. Cox regression analysis was performed to identify predictors of bPFS. Toxicity was assessed using CTCAE (V.4).
Results: A total of 31 metastases were treated. Localizations were pelvic lymph nodes (n=21), and bones (n=9) of which were pelvic bones (n=4), ribs and sternal (n=3), vertebral body (n=2). One patient had a metastasis of the penis (n=1). The median follow-up was 13.5 months (3–48) with an overall survival of 100%. A treatment response was detected in 18/20 (90%) patients. The median PSA of 1.47ng/ml (0.38–8.82) prior to MDT decreased significantly to 0.20 ng/ml (0.0–1.93) after treatment (p<0.001). For the entire cohort (n=20) median bPFS was 14 months (9.3–18.7). Patients with solitary metastases had a significant longer bPFS with mean 20.2 months (10.6–29.9) compared to 9.7 months (4.9–14.6) for those with 2-3 metastases (p=0.037). Furthermore, patients without additional ADT and 2-3 metastases in 68Ga-PSMA PET/CT had a significant higher risk for PSA progression after MDT (HR 10.928, CI 1.053–113.421, p=0.045). Of 20 patients 9 (45%) had acute toxicity I° and 1 patient (5%) also experienced acute toxicity II°. Late toxicities I° occurred in 1 patient (5%). No acute or late toxicities >II° were observed.
Conclusion: Using PSMA-PET/CT guided RT for MDT of OPCa led to a significant decrease in PSA-levels with minimal therapy associated toxicity. Especially patients with solitary metastases may benefit from this treatment approach. However, randomized trials with larger patient collectives are necessary.
Keywords: prostate cancer, oligometastasis, metastasis directed therapy, radiotherapy, 68 GA-PSMA-PET/CT, biochemical recurrence, radiotherapy toxicity
PCa, prostate cancer; OPCa, oligiometastatic prostate cancer; bPFS, biochemical progression free survival; GS, gleason score; PSA, prostate specific antigen; PSA DT, prostate specific antigen doubling time; MDT, metastasis directed therapy; ADT, androgen deprivation therapy; PSMA, prostate specific membrane antigen; GTV, gross tumor volume; PTV, planning treatment volume; VMAT, volumetric arc (radio)therapy; Gy, Gray; RT, radiotherapy; RP, radical prostatectomy
In 1995 Hellman and Weichselbaum first described oligometastasis as an intermediate state between localized primary and widespread metastatic disease.1 Since then, there is growing evidence that oligometastatic cancer shows a different biological behavior, indicating that the tumor has not yet reached the full metastatic potential.2 Consequently, local ablative therapy of primary disease and metastases alone might be a sufficient treatment option for this subgroup of patients.2 This concept has already led to a change in the management of metastasized tumors like colorectal3–5 or renal cancer,6 where patients with singular or a low number of metastases are still considered to be curable by local treatment (Metastasis Directed Therapy=MDT). Accordingly, OPCa patients with one to three metastases receiving systemic therapy showed a better prognosis with increased overall and cancer specific survival compared to patients with widespread disease.7 Several retrospective studies suggested that in this subgroup of patients MDT could delay disease progression and palliative androgen deprivation therapy (ADT).8 Recently published data of a phase II randomized prospective trial by Ost et al.9 confirmed that MDT in OPCa patients can at least prolong ADT free survival (21 vs. 13 months) when compared to surveillance.9 Moreover, there was no difference regarding the quality of life between both arms and only a low number of patients in the MDT arm developed grade 1 toxicity.9 Hence, MDT causes only minor therapy associated toxicity but can prolong survival time without side effects of systemic therapy.
There are some limitations, however, that makes it difficult to establish MDT for OPCa in clinical practice. Firstly retrospective studies included very inhomogeneous patient groups with different definitions of oligometastatic state, and secondly the study endpoints were often inconsistent.8 Furthermore, most of the studies, including the phase II trial of Ost et al.,9 used a Choline-PET/CT for diagnosis of oligometastasis.8,9 Despite the fact that Choline-PET/CT and whole-body MRI have improved detection rates of metastatic disease in patients with biochemical recurrence after initial curative treatment, the 68 Ga-PSMA PET/CT showed a significant higher sensitivity and specificity for prostate cancer metastases.10–12 We therefore assume that 68Ga-PSMA-PET/CT could further improve outcome of MDT by differentiation of “true” oligometastatic patients and those who suffer from widespread occult metastasis with only few visible in standard imaging modalities.13 Hence, we analyzed the PSA outcome after curative RT as MDT of OPCa patients staged by 68Ga-PSMA-PET/CT.
Study design
This study was approved by the local Ethics Committee of the University of Marburg (AZ175/18). A total of 31 prostate cancer patients who had undergone MDT between 2015 and 2018 at university hospital Marburg, Germany, were identified. The choice of MDT was made after consultation with a multidisciplinary team and the patients. Data have been collected from routine check-up documentations after MDT. Of these 31 patients, 20 met the inclusion criteria of the study (Figure 1).
Inclusion criteria were
PSA progression free survival (bPFS) after MDT as well as treatment related toxicity was evaluated.
Radiotherapy
All patients underwent a CT-based treatment planning in supine position with 3mm slice thickness (Siemens, Healthineers, Germany). Contouring and treatment planning was performed using Eclipse™ Treatment Planning System (Varian medical systems, Palo Alto, California, USA). GTV delineation took place after image fusion with 68Ga-PSMA-PET/CT and MRI, considering all available clinical and histopathological information. GTV to PTV margins depended on the irradiated site (7-10 mm for bone metastasis, 3-7 mm for lymph node metastases). Organs at risk were delineated depending on the irradiated region. All patients were treated with image-guided RT (IGRT).9 patients received 3D conformal RT, whereas 10 patients were treated using VMAT. Especially patients with lymph node metastases were treated with VMAT. A cone-beam CT was performed before each fraction in case of VMAT. For 3D conformal RT weekly megavoltage image acquisition with fusion of the reference image was performed. All metastases were treated with biologically equivalent doses of 100–148 Gray (α/β=2). 8 of 13 patients with lymph node metastases received additional irradiation of internal and external iliac lymphatics with 50.4 to 59.4 Gy with a single dose of 1.8 Gy per fraction. Positive lymph nodes in 68Ga-PSMA-PET/CT received simultaneous boosts with 69.3 Gy with a single dose of 2.1 Gy per fraction. Therefore, the most common fractionation scheme was 33x2.1 Gy for lymph node metastases. For bone metastases the most common fractionation scheme was 30x2 Gy. One patient received brachytherapy on a metachronous pararectal lymph node with a total dose of 27 Gy in three fractions.
Follow-up
Clinical examination was performed weekly during RT. The first radio-oncological follow-up visit took place 6 weeks after RT and every 6 months thereafter. Toxicity was assessed using the Common Terminology Criteria for Adverse Events version 4.0 (CTCAE). Furthermore, periodical urological follow-up with routine assessment of PSA values was performed every three months.
Evaluation of PSA-outcome
Biochemical response to MDT was defined as a decrease in PSA levels of 25-50% after MDT. Patients with increasing PSA levels after treatment were classified as non-responding to MDT. The bPFS was defined as time interval from start of MDT to first increase in PSA serum levels of 0.2ng/ml above the nadir. Patients with decreasing PSA levels that did not reach the nadir during observation period or stable PSA values were considered as non-progressors.
Statistical analysis
Statistical analysis was performed using SPSS version 21 (IBM Corporation, Somer, NY, USA). The median difference between PSA values prior to MDT and after treatment was compared using the Wilcoxon signed rank test. Clinical and pathological variables were dichotomized as follows: ≤T2c vs. ≥T3, Gleason Score (GS) ≤7a vs. ≥7b, PSA ≤10 vs. ≥10, No. of metastasis 1 vs. 2-3, PSA at MDT ≤1.47 vs >1.47, PSA DT ≤6 months vs >6 months, time between PCa diagnosis and MDT ≤24 months vs. >24 months, bone and others vs. lymph node (localization of metastasis), intrapelvic vs. extrapelvic metastases (pattern of metastasis), ADT at MDT yes vs. no and age at MDT ≤67 vs >67. Associations between categorical variables and biochemical progression were assessed by Fisher’s exact test, and two-sided P-values <0.05 were considered to be statistically significant. Median bPFS was calculated using Kaplan Meier estimators, and, after stratifying by clinical and pathological parameters, differences in bPFS were compared using log rank tests. Categorical variables which showed a significant association with bPFS were included in a cox regression model. Their impact upon patients’ bPFS was expressed as hazard ratio (HR) provided with 95% confidence intervals.
Study population
The median age at diagnosis of metastasis was 67 (52–87) and the median time from initial PCa diagnosis to first detection of metastasis was 24 months (0–180). The median PSA prior to MDT was 1.47 (0.38–8.82) and the median PSA doubling time (PSA DT) prior to MDT was 6.1 months (1.0–19.2). A total of 8 (40%) patients received ADT during or after MDT. For ADT, LHRH agonists and oral Bicalutamide or Flutamide were applied. Initially 15 (75%) patients were treated by RP and 5 (25%) by RT at initial diagnosis. After RP 5 (25%) patients with R1 resection received adjuvant RT of the prostate bed. Furthermore, 5 (25%) patients received an initial course of ADT concomitant to the treatment of the primary. A total of 14 (70%) patients had high risk cancer, 4 (20%) had intermediate risk and 2 (10%) had low risk cancer according to D’Amico et al14 The clinical and pathological data are summarized in Table 1.
Parameter |
n |
|
Patients included |
20 |
|
Age at diagnosis of metastasis |
Median (range) |
67 (52–87) |
Time between PCa diagnosis and metastasis (months) |
Median (range) |
24 (0–180) |
Primary tumor classification |
≤T2c |
5 |
≥T3 |
14 |
|
Not available |
1 |
|
Nodal status at PCa diagnosis |
N0 |
19 |
N1 |
1 |
|
Distant metastasis at PCa diagnosis |
M0 |
19 |
M1 |
1 |
|
Gleason Score |
≤6 |
4 |
7a |
5 |
|
7b |
4 |
|
8−10 |
7 |
|
PSA at PCa diagnosis (ng/ml) |
<10 |
7 |
10-20 |
4 |
|
>20 |
8 |
|
Not available |
1 |
|
Type of curative treatment at PCa diagnosis |
Surgery |
15 |
Radiotherapy |
5 |
|
PSA at MDT (ng/ml) |
Median (range) |
1.47 (0.38–8.82) |
PSA DT prior to MDT (months) |
Median (range) |
6.1 (1–19.1) |
No. of metastases |
1 |
11 |
2 |
7 |
|
3 |
2 |
|
Location of metastases |
nodal |
11 |
bone |
7 |
|
nodal+bone |
1 |
|
nodal+other |
1 |
|
ADT additional to MDT |
No |
12 |
Yes |
8 |
|
Follow up after MDT (months) |
Median (range) |
13.5 (3–48) |
Table 1 Patients and Tumor characteristics
MDT, metastasis directed therapy; ADT, androgen deprivation therapy; Pca, prostate cancer; PSA, prostate specific antigen; PSA DT, PSA doubling time
Patterns of metastasis and treatment response
The median follow-up was 13.5 months (3–48) and no patient died during observation period. A total of 31 metastases were treated. Localizations were pelvic lymph nodes (n=21), pelvic bones (n=4), ribs and sternal (n=3) vertebral body (n=2) and other organs (n=1). Nodal metastases were found in 11 patients, 7 patients had bone metastases and 1 patient showed bone and lymph node metastasis. Another patient had a nodal metastasis and a solitary metastasis of the penis. Of 20 patients, 17 (85%) showed a decrease of at least 25-50% in PSA levels after MDT. One patient (5%) showed no decrease in PSA levels after MDT. Considering that this patient had increasing PSA levels (0.02 to 0.77ng/ml) prior to MDT and remained stable during follow up without additional ADT we nevertheless classified him as responder to MDT. Only 2 (10%) patients were classified as non-responders with continuously rising PSA values after MDT. For the entire cohort, the median PSA value at the beginning of MDT was 1.47 ng/ml (0.38–8.82), compared to 0.20 ng/ml (0–1.93) after treatment (p<0.001). When only patients without additional ADT were analyzed (n=12) the median PSA value at the beginning of MDT was 1.3 ng/ml (0.38–4.10) compared to 0.20ng/ml (0.03–1.93) after treatment (p=0.010). For patients with additional ADT the median PSA at MDT was 2.25ng/ml (0.50–8.82) compared to 0.27 ng/ml (0.0–1.23) afterwards (p=0.012, Figure 2).
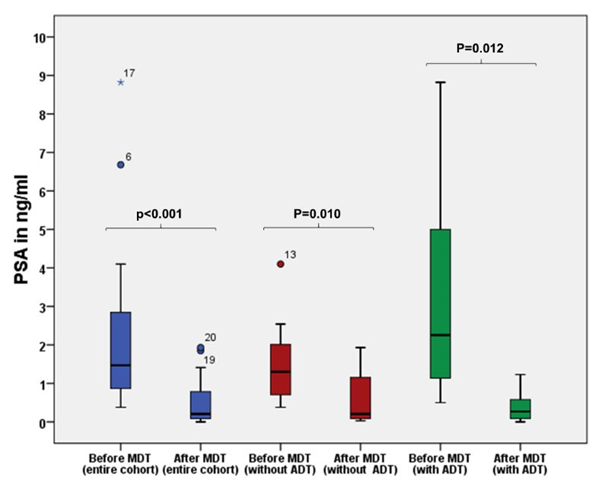
Figure 2 Comparison of PSA value prior to MDT and after treatment for the entire cohort (blue boxes), patients without additional ADT (red boxes) and patients with additional ADT (green boxes). P-values for the Wilcoxon signed rank test.
Of all 20 patients 12 (60%) showed a biochemical progression during the observation period. Median bPFS for the entire cohort (n=20) was 14 months (9.3–18.7) (Figure 3A). To further investigate the influence of ADT on MDT outcome we analyzed the bPFS of those patients without additional ADT to MDT (n=12) versus with additional ADT (n=8). In the subgroup without ADT the median bPFS was 12 months (4.8–19.2) (Figure 4A) whereas in the subgroup with additional ADT the median bPFS was 14 months (6.6–21.4). Moreover, several patients showed continuously declining PSA values at the time of this report.

Figure 3 (A) bPFS for the entire cohort. (B) bPFS stratified by the number of metastases in 68 Ga-PSMA PET/CT for the entire cohort. P-value for the log rank test.
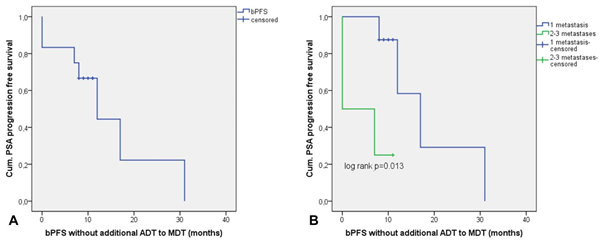
Figure 4 (A) bPFS for the subgroup of patients without additional ADT to MDT. (B) bPFS stratified by the number of metastases in 68Ga-PSMA PET/CT for the subgroup without additional ADT to MDT. P-value for the log rank test.
Factors predicting bPFS
We found a significant association between the number of metastases and PSA progression after MDT. 8/12 (66.6%) patients with PSA progression after MDT had 2-3 metastases, whereas 7/8 (87.5%) of the non-progressors showed a solitary bone or lymph node metastasis (p=0.028, Table S1). In line with this, patients with a solitary metastasis had a significant longer bPFS with mean 20.2 months (10.6–29.9) compared to 9.7 months (4.9–14.6) for those with 2-3 metastases (p=0.037, Table 2, Figure 3B). In support of these results, a low PSA value of <1.47 ng/ml prior to MDT, indicating a lower tumor burden, showed a trend towards a longer bPFS although this result didn’t reach statistical significance (p=0.060, Table 2). Tumor stage, PSA at PCa diagnosis, PSA DT, GS, age at diagnosis of metastasis, localization of metastasis (lymph node vs. bone and other), pattern of metastasis (intrapelvic vs. extrapelvic metastases), time between initial PCa diagnosis, first metastasis and additional ADT to MDT showed no significant associations with PSA progression (Table S1) and bPFS (Table 2). When these factors were analyzed in patients without additional ADT to MDT (n=12) again only the number of metastasis showed a significant association with bPFS. In this subgroup, patients with a solitary metastasis had a significant longer bPFS of mean 18.5 months (8.8–28.2) compared to 4.5 months (0–9.1) for those with 2-3 metastasis (p=0.013, Figure 4B). In cox regression analysis, patients with 2-3 metastases in 68Ga-PSMA PET/CT without additional ADT had a significantly higher risk of PSA progression after MDT (HR 10.9, CI 1.053–113.421, p=0.045).
Parameter |
Mean bPFS (mo) |
95% CI |
P* |
Tumor stage |
|||
≤T2c |
11.8 |
4.2–19.3 |
0.661 |
≥T3 |
15.4 |
8.8–21.9 |
|
PSA at PCa diagnosis |
|||
≤10 ng/ml |
11.8 |
8.8–14.8 |
0.767 |
>10 ng/ml |
14.3 |
7.4–21.2 |
|
Gleason Score |
|||
≤7a |
13.6 |
9.5–17.7 |
0.959 |
>7a |
14.5 |
6.8–22.3 |
|
Age |
|||
≤67 |
16.9 |
9.2–24.8 |
0.294 |
>67 |
11.6 |
6.7–16.6 |
|
PSA at MDT (median) |
|||
<1,47 ng/ml |
19.1 |
9.9–28.3 |
0.06 |
>1,47 ng/ml |
10.2 |
5.5–14.8 |
|
PSA DT |
|||
≤6 Monate |
14.5 |
11.6–17.4 |
0.564 |
>6 Monate |
12.9 |
5.9–20.0 |
|
No. of metastases |
|||
1 |
20.2 |
10.6–29.9 |
0.037 |
02-03 |
9.7 |
4.9–14.6 |
|
Localization of metastases |
|||
nodal |
12.2 |
8.6–15.9 |
0.285 |
bone + other |
18.4 |
7.1–29.6 |
|
Pattern of metastasis |
|||
Intrapelvic |
11.9 |
8.2–15.5 |
0.184 |
extrapelvic |
20.4 |
8.1–32.6 |
|
ADT to MDT |
|||
yes |
14.6 |
6.4–22.7 |
0.973 |
no |
13.9 |
9.6–18.2 |
|
Time between PCa diagnosis and metastasis (months) |
|||
≤24 |
|||
>24 |
18.6 |
10.6–26.5 |
0.095 |
10.1 |
5.0–15.1 |
||
Table 2 Factors predicting bPFS
*P-values for the log rank test
MDT, metastasis directed therapy; ADT, androgen deprivation therapy; Pca, prostate cancer; PSA, prostate specific antigen; PSA DT, PSA doubling time
Parameter |
Progressors |
Non-progressors |
P* |
Tumor stage |
|||
≤T2c |
4 (33.4%) |
1 (14.3%) |
0.603 |
≥T3 |
8 (66.6%) |
6 (85.7%) |
|
PSA at PCa diagnosis |
|||
≤10 ng/ml |
3 (27.3%) |
4 (50%) |
0.377 |
>10 ng/ml |
8 (72.7%) |
4 (50%) |
|
Gleason Score |
|||
≤7a |
5 (41.7%) |
4 (50%) |
1 |
>7a |
7 (58.3%) |
4 (50%) |
|
Age |
|||
≤67 |
6 (50%) |
5 (62.5%) |
0.67 |
>67 |
6 (50%) |
3 (37.5%) |
|
PSA at MDT (median) |
|||
<1.47 ng/ml |
4 (33.4%) |
6 (75%) |
0.17 |
>1.47 ng/ml |
8 (66.6%) |
2 (25%) |
|
PSA DT |
|||
≤6 Monate |
4 (33.4%) |
6 (75%) |
0.17 |
>6 Monate |
8 (66.6%) |
2 (25%) |
|
No. of metastases |
|||
1 |
4 (33.4%) |
7 (87.5%) |
0.028 |
02-03 |
8 (66.6%) |
1 (12.5%) |
|
Localization of metastases |
|||
nodal |
8 (66.6%) |
4 (50%) |
0.648 |
bone + other |
4 (33.4%) |
4 (50%) |
|
Pattern of metastasis |
|||
Intrapelvic |
9 |
6 |
1 |
extrapelvic |
3 |
2 |
|
ADT to MDT |
|||
yes |
7 (58.3%) |
5 (62.5%) |
1 |
no |
5 (41.7%) |
3 (37.5%) |
|
Time between PCa diagnosis and metastasis (months) |
|||
≤24 |
|||
>24 |
5 (41.7%) |
6 (75%) |
0.197 |
7 (58.3%) |
2 (25%) |
||
Table S1 Associations between clinicopathological parameters and PSA progression after MDT
P* values for Fisher’s Exact Test
Toxicity
Regarding therapy associated toxicity we observed urogenital and gastrointestinal side effects in form of urinary urgency, dysuria and diarrhea/constipation for those patients who received radiotherapy to pelvic lymph nodes or pelvic bones (n=16). Of these 16 patients, 5 (31.2%) showed acute urogenital toxicity I°, 1 patient (6.2%) acute urogenital toxicity II°, and 7 patients (43.7%) acute gastrointestinal toxicity I°. Late toxicities occurred in 1 patient (6.2%), which were urogenital and gastrointestinal I°, in form of chronic irregular bowel movement and urinary urgency. Of 8 patients which received additional irradiation of internal and external iliac lymphatics 6 (75%) had acute urogenital and/or gastrointestinal toxicities I° and 1 patient (12.5%) acute urogenital toxicity II°. The 4 patients which received radiotherapy to ribs, sternum and vertebral bodies had no therapy associated toxicity. In summary, of all 20 patients 9 (45%) experienced acute toxicity I°, 1 patient (5%) had also acute toxicity II° and only 1 (5%) were noted to have late toxicities. No acute or late toxicities >II° were observed.
There is growing evidence that oligometastatic cancer has a different biology, with differently expressed genes, compared to polymetastatic cancer.15,16 For pancreatic cancer, Yachida et al.17 showed that gaining metastatic capacity is a matter of time, with oligometastatic cell clones emerging before polymetastatic clones.17 Furthermore, metastasis to metastasis spread by polyclonal seeding was described for prostate cancer.18 Thus, for a successful MDT, metastases must be detected with high sensitivity and specificity in an early state of the metastatic cascade. The advantage of PSMA-PET/CT lies in a high detection rate at low PSA levels, as seen in early recurrent prostate cancer. A systematic review of Perera et al.12 revealed detection rates for biochemical recurrent PCa of 42%, 58%, 76% and 95% for PSA values 0–0.2, 0.2–1, 1–2 and >2 ng/ml.12 These results were confirmed by Rauscher et al.19 with detection rates for recurrent prostate cancer (lymph nodes, local recurrences and bone metastases) of 74% at low PSA values (0.5–1.0 ng/ml) and even 55% in patients with very low PSA values (0.2–0.5 ng/ml).19 We therefore assumed a sufficient detection rate of PSMA-PET/CT for our patient collective with a median PSA of 1.47ng/ml before MDT. Most of the retrospective studies and randomized prospective trials (STOMP, ORIOLE) regarding OPCa were not based on PSMA-PET/CT but rather on conventional imaging or 11-Choline-PET/CT.9,18,20,21 There is a risk of understaging primary or recurrent PCa due to a significantly lower sensitivity in detecting metastases with 11-Choline-PET/CT.10 A direct comparison of 11-Choline- and PSMA-PET/CT revealed a significantly higher detection rate of PSMA-PET/CT for lymph node as well as bone metastases in patients with biochemical recurrent PCa.22 Furthermore, lymph node metastases detected by PSMA-PET/CT were significantly smaller than those detected by 11-Choline-PET/CT (6mm vs. 11.7mm).22 Consequently, there were significant differences in the detection rate of OPCa, and patients who were classified as oligometastatic in 11-Choline-PET/CT were frequently upstaged to polymetastatic after PSMA-PET/CT.22
Nevertheless, to our knowledge, only few studies have investigated the efficacy of MDT based on PSMA-PET/CT-imaging in OPCa.23–25 In our present retrospective study on PSMA-PET/CT-guided RT as MDT, we found a significant decrease in PSA levels of median 1.47ng/ml prior to RT down to median 0.20ng/ml at last follow up (p<0.001). This was in line with the results of Guler and Henkenberens et al.23,24 who found similar levels of PSA response to MDT in OPCa diagnosed by PSMA-PET/CT.23,24 However, in the study of Guler et al.23 all patients also had ADT throughout treatment and in the study of Henkenberens et al.24 patients with bone metastases continued ADT during and after radiotherapy.23,24 Considering that 8 of 20 patients (40%) in our present study also received ADT during and after RT, we analyzed the PSA response separately in the subgroup with (n=8) and without (n=12) additional ADT (Figure 2). Thus, we were able to show a PSA response to MDT that was not influenced by the effects of ADT, with a median PSA of 1.3ng/ml (0.38–4.10) before compared to 0.20ng/ml (0.03–1.93) after treatment. As expected, the highest decrease in PSA level was found in patients with additional ADT with a median PSA of 2.25ng/ml (0.50–8.82) prior to MDT compared to 0.27ng/ml (0.0–1.23) afterwards. The follow-up time in our study was median 13.5 months with a bPFS of 14 months (9.3–18.7). Even when only patients without concomitant ADT (n=12) were analyzed, a similar bPFS of median 12 months (4.8–19.2) was detected. This result surpasses the median bPFS of 10 months after MDT in the prospective 11-Choline-PET/CT-based STOMP trial, in which patients were treated by surgery or SBRT and the definition of PSA progression was not as strict as in our study. Supporting our results Kneebone et al.25 found a median bPFS of 11 months in 57 PSMA-PET/CT-confirmed OPCa patients treated only by SBRT with a follow-up time of median 16 months.25 None of their 57 patients received systemic treatment combined with RT, and PSA progression was also defined as nadir + 0.2ng/ml.
However, it must also be considered that patients included in the STOMP trial and in the study of Kneebone et al.25 received SBRT with high doses per fraction (6 to 20 Gy), which was less comparable to our mostly conventionally fractionated RT. The fact that bPFS in our study was similar to that of both other studies suggests that both fractionation schedules seem to be equivalent in terms of bPFS. In addition, the treatment-related toxicity in our study was equal to previously published studies, with no acute or late toxicity >II° having occurred. Identifying factors that predict sustained therapy response is a necessary prerequisite for successful implementation of MDT in clinical routine. In this context we could show that patients with solitary metastases had a significantly longer bPFS of mean 20.2 months (10.6–29.9) compared to mean 9.7 months (4.9–14.6) for patients with 2-3 metastases (p=0.037). Of note, 6 of 11 patients (54%) with solitary metastases had bone metastases. The number of metastases in patients without additional ADT was also a significant predictor for bPFS in cox regression analysis (HR 10.9, CI 1.053–113.421, p=0.045). To our knowledge, previous PSMA-PET/CT based studies of treating OPCa with MDT weren’t able to show a significant association between bPFS and the number of metastases. Nevertheless, it must be considered that PCa patients with limited metastases may have an inherently better prognosis. Concerning this, Ost et al.26 could show that a lower number of metastases was associated with an improved prostate cancer specific survival.26
Our findings that a lower PSA level of <1.47 ng/ml prior to MDT showed a trend towards a longer bPFS after treatment supports the hypothesis that patients with a lower tumor burden and a lower number of metastases are more suitable for MDT therapy concepts. In contrast to the PSA level prior to MDT and the number of metastases, bPFS was not affected by initial tumor stage, GS, initial PSA-value as well as PSA-DT prior to MDT, age, additional ADT to MDT, time to first presentation of metastasis, pattern of metastasis and localization of metastases. Despite our promising results, our study has some limitations. Firstly, the retrospective nature may lead to a selection bias. Secondly, with 20 patients included, the study collective was too small to deduct reliable results. Thirdly, although all patients were treated with RT, the fractionation schedules, total doses and treatment volumes were heterogenous (8 patients received additional RT of internal and external iliac lymphatics) and finally, 40% of the patients were treated with additional ADT to MDT.
In this study we could demonstrate a high treatment efficacy of PSMA-PET/CT-guided RT as MDT in OPCa patients. Especially patients with a low number of metastases (solitary lymph node or bone metastasis) and low PSA values at initial diagnosis of metastasis (<1.47ng/ml) seemed to benefit from MDT. With its high sensitivity and specificity in detecting PCa metastases, even at low PSA levels, the PSMA-PET/CT is particularly suitable for identifying those patients. However, randomized prospective trials with larger patient collectives are necessary to implement MDT for OPCa as a standard treatment option in clinical routine.
Ethics approval and consent to participate
Approval from the local ethics committee of the Phillips University of Marburg (10.12.2018). Prof. Dr. Gerd Richter (Vorsitzender). (AZ Studie 175/18).
Consent for publication
Not applicable.
Availability of data and materials
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
The authors declare that they have no competing interests.
None.
All authors read and approved the final manuscript.
Sabine Fischer (Study Nurse)

©2020 Dumke, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.