International Journal of
eISSN: 2574-8084


Case Report Volume 5 Issue 6
Department of Radiology, VM Medical Park Hospital, Turkey
Correspondence: Inci Kizildag Yirgin, Department of Radiology, VM Medical Park Hospital, 41140 Kocaeli, Turkey, Tel 5308204689
Received: October 24, 2018 | Published: November 16, 2018
Citation: Kizildag YI. Primary renal lymphoma: CT findings of a rare case. Int J Radiol Radiat Ther. 2018;5(6):313-316. DOI: 10.15406/ijrrt.2018.05.00185
Primary renal lymphoma (PRL) is an extremely rare disease. Due to the relative rarity of PRL, few studies have classified the imaging patterns of the disease. We describe computed tomography (CT) findings of a patient with PRL, and discuss the differential imaging features. PRL is typically large, hypovascular, infiltrative solid mass that does not invade the vessels. Having an understanding of typical imaging findings is very important for accurate diagnosis, differentiation from other renal malignancies, and a correct decision to biopsy.
Keywords: kidney, lymphoma, imaging, CT
PRL, primary renal lymphoma; CT, computed tomography; SRL, secondary renal lymphoma; RCC, renal cell carcinoma; DLBCL, diffuse large cell B-cell lymphoma; IVC, inferior vena cava inferior
Primary renal lymphoma (PRL) is an extremely rare disease.1 The rate of renal lymphoma in all cases of extranodal NHL is only 1%.2 Unlike secondary renal lymphoma (SRL), PRL does not accompany widespread nodal or extranodal lymphoma.3 Due to the relative rarity of PRL, few studies have classified the imaging patterns of the disease.4–6 Case reports and case series from urologic and oncologic literature suggest an imaging spectrum similar to that of SRL.7 Although the diagnosis of PRL can be challenging, having a better understanding of imaging findings can help clinicians differentiate lymphoma from other renal malignancies such as renal cell carcinoma (RCC). Distinguishing between these two diseases is critical because renal lymphoma is treated with chemotherapy, whereas RCC typically requires surgery or ablation. In this paper, we describe the clinical and computed tomography (CT) findings of a patient with primary diffuse large cell B-cell lymphoma (DLBCL), and discuss the unique imaging features characteristic to this disease entity.
A 76-year-old woman presented with chronic lower back pain. Her past medical history was remarkable for hypertension and peptic ulcer disease. On physical examination, there was a palpable mass in the right lumbar region. Routine blood tests revealed elevated levels of urea and creatinine, at 50mg/dl (normal 15-40mg/dl) and 1.5 mg/dl (normal 0.57-1.11mg/dl), respectively. Urinalysis revealed a microscopic hematuria.
Ultrasonography of the kidneys demonstrated a solid mass in the right kidney. The right kidney itself was enlarged with a thin cortex, but had no signs of pelvicalyceal obstruction. The left kidney appeared within normal limits. A follow-up CT scan of the abdomen was performed, revealing a soft-tissue mass in the right renal parenchyma measuring 17x12x10cm. The perirenal area was completely infiltrated by the mass, while the adjacent colon segments and posterior psoas muscles also demonstrated partial infiltration (Figure 1). The aortocaval lymph node was mildly enlarged. There was no evidence of extension into the renal artery or vein upon administration of IV iodinated contrast. The tumor was in close proximity with the inferior vena cava inferior (IVC) and abdominal aorta, but there was no evidence of tumor invasion or tumor thrombus in these structures. Nephrogram and pyelogram phases in the right kidney were significantly delayed compared to the left. Additional imaging studies, including CT scans of the thorax and brain, did not reveal any metastatic lesions. The size of the mass was concerning with respect to malignancy, decreasing the likelihood that this was a benign lesion such as oncocytoma, adenoma, or angiomyolipoma. Furthermore, the patient had no signs of, or risk factors for, urothelial carcinoma. Given that RCC is the most common cause of solid renal masses in elderly patients, and that radiologic findings were consistent with this diagnosis, the patient was admitted to hospital and booked for a right nephrectomy, right hemicolectomy, and lymph node dissection.
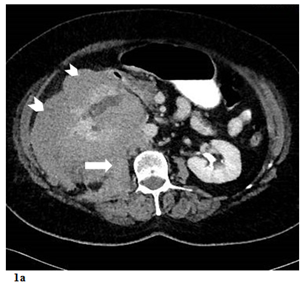
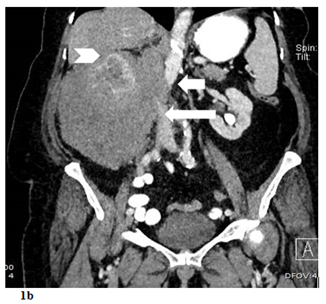
Figure 1 a) Contrast enhanced axial CT scan demostrating hypodense renal mass (arrowheads) involving the right kidney with perinephric area and psoas muscle (arrow) infiltration. b) Contrast enhanced coronal CT scan demostrating mass effect on the liver (arrowhead), VCI (long arrow) and aorta (short arrow).
Pathology of the surgical specimen was noted to be renal parenchyma, capsule, and perirenal regions infiltrated with a white-gray tumor possessing a “hard-elastic composition”. Adjacent colon segments measuring 13cm long and 9cm thick were removed, with evidence of mucosal, submucosal, and serosal tumor infiltration. Two enlarged lymph nodes had infiltration by malignant cells. Multiple tumor sections revealed lymphomatous tissue composed of centrocytes and centroblasts in a diffuse growth pattern. Using immunohistochemical testing, the malignant cells were positive for CD20 and CD3, but negative for CK. The final pathological diagnosis was diffuse B-cell follicle center lymphoma.
The patient was urgently referred to the medical oncology department for further staging and chemotherapy. At present, the use of rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisolone (R-CHOP) constitutes the standard treatment for intermediate or high grade primary renal lymphoma. She was treated with 3 cycles of R-CHOP chemotherapy. At 6 months follow-up, there were no pathological findings at the surgical site or elsewhere in the body on CT scan (Figure 2). Thus far, the patient has been followed for 8 months with no recurrence in her disease.
Lymphamatous infiltration of the kidney commonly occurs in the form of SRL, with widespread nodal or extranodal lymphoma. In contrast, PRL rarely involves the kidneys alone.3,8 To avoid misdiagnosis, various guidelines [sources] have reported the following diagnostic criteria for PRL, including (1) disease localized to the kidney without evidence of other organ or nodal involvement, (2) renal failure in the absence of other causes, with rapid improvement of renal function after treatment of lymphoma, or (3) diagnosis of PRL confirmed by biopsy.9 In our case, there was limited involvement in only one kidney, so there was no significant renal impairment; diagnosis was subsequently confirmed with biopsy.
Although any lymphoma subtype can affect the kidneys, the most common form of renal lymphoma (both PRL and SRL) is non-Hodgkin lymphoma, typically DLBCL.10–12 PRL may present as a solitary or multifocal mass, an ill-defined infiltrative lesion engulfing the kidney, or as diffusely enlarged kidneys.2,7,13–15 On ultrasound, renal lymphoma is usually hypoechoic or anechoic.16 Diffuse nephromegaly may also be seen. Sonographic findings are generally nonspecific and require further investigation with CT or MRI.
PRLs are often large infiltrative renal tumors that extend into the perinephric space and retroperitoneum. However, despite being an aggressive tumor, PRL rarely involves the IVC. This characteristic of PRL can help in distinguishing it from RCC.17,18 Renal lymphoma can exert a mass effect on adjacent vascular structures and soft tissues, but will not invade them. Since RCC will invade adjacent vascular structures, it is very important to identify this feature on CT and be aware of this when generating a differential diagnosis.
PRL occurring purely as a renal sinus mass is extremely rare.19 Careful evaluation to ensure that the epicenter of the tumor is not within the collecting system is helpful in differentiating PRL from urothelial carcinoma. However, definitive diagnosis usually requires biopsy.20 Renal lymphoma is typically a hypovascular tumor. This feature can help differentiate it from more common hypervascular tumors such as RCC, oncocytoma, and angiomyolipoma.7
Contrast enhanced CT is the first choice of imaging modality to detect lesions. CT scan is also useful in disease staging, or in monitoring post-operative and post-treatment status. MRI can be useful in patients with a history of renal insufficiency, allergy to contrast dye, or when there is a heightened concern for radiation exposure such as with children and young adults. The role of PET/CT in renal lymphoma has not been formally evaluated in any large-scale studies to date.21,22 However, PET/CT may be useful in distinguishing between lymphoma and RCC since the former is intensely FDG-avid while the latter may not show as much FDG uptake.23
Traditionally, PRL has been associated with a poor prognosis.16,24–26 However, early diagnosis and proper management results in a favorable outcome. Recent reports suggest that early diagnosis and administration of R-CHOP chemotherapy can substantially improve renal function within 2–4 weeks, and may improve overall 5-year survival rates.27,28 Morel et al.,29 reported that tumor size larger than 10cm, involvement of the renal hilum, and diffuse renal infiltration are associated with a worse prognosis.29
PRL is typically a large, hypovascular, infiltrative solid mass that does not invade the vessels. Having an understanding of typical imaging findings is very important for accurate diagnosis, differentiation from other renal malignancies, and formulation of a correct treatment approach. Using this framework, a correct decision to biopsy can be made while avoiding unnecessary nephrectomy.
None.
The author declares that there is no conflict of interest.

©2018 Kizildag. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.