International Journal of
eISSN: 2574-8084


Clinical Paper Volume 6 Issue 3
1Genesis Cancer Care Victoria, Ringwood, Australia
2The Austin Hospital, Heidelberg, Australia
3University of Melbourne, Melbourne, Australia
4Monash University, Australia
5Royal Marsden Hospital, London, UK
Correspondence: Michael Chao, Genesis Cancer Care Victoria, 36 Mt Dandenong Road, Ringwood East, Victoria 3135, Australia, Tel 61-03-88703300
Received: May 16, 2019 | Published: May 30, 2019
Citation: Chao M, Ong WL, Joon DL, et al. Post Prostatectomy Radiotherapy: Can the urologist help to reduce rectal toxicity? Int J Radiol Radiat Ther.2019;6(3):87-92. DOI: 10.15406/ijrrt.2019.06.00224
Purpose: To report on feasibility of hydrogel spacer (HS) insertion by for patients undergoing post prostatectomy radiotherapy (PPRT) based on our institutional experience.
Materials and methods: Four consecutive patients referred for PPRT who had radiological (prostate specific membrane antigen positron emission tomography and/or magnetic resonance imaging scans) or histological evidence of local recurrence were considered for HS insertion. The HS was inserted into the plane between the local recurrence and anterior rectal wall. Pre-HS and post-HS computerised tomography planning scans were performed, and the rectal dosimetry for both planning scans was compared. The dose to the radiological or histological confirmed local recurrence was escalated to 75.6Gy in 1.8 Gy fractions, with 67.2Gy in 1.6 fractions to the rest of the post prostatectomy bed using intensity modulated radiation therapy.
Results: All 4 patients successfully underwent their HS insertion without postoperative complications. The distance between the rectal wall and local recurrence was increased from immediate vicinity to a mean of 12mm after HS insertion. The HS had an influence on all rectal dose endpoints with a significant 51% reduction in the mean percentage of rectal volume receiving 70Gy (21.1 pre-HS vs 10.4 post-HS). Only 1 patient developed common terminology criteria for adverse events grade 1 acute gastrointestinal toxicity despite dose escalated PPRT.
Conclusion: We report one of the first series on the feasibility of HS insertion for PPRT, with significant reduction in the high dose rectal volume dosimetry, and hence allows for safer dose escalation to the target volume. The use of HS in the PPRT setting by urologists can help in reducing GI toxicity.
Radiotherapy is used in the post-prostatectomy setting, as an adjuvant treatment for patients at high risk of local recurrence,1−3 or as salvage treatment in patients with biochemical failure.4,5 However, some clinicians may argue against indiscriminate use of post prostatectomy radiotherapy (PPRT), especially in the adjuvant setting, given that PPRT is not without morbidities such as rectal toxicities. While late grade 1-2 rectal toxicities are not uncommon, the risk of late grade 3-4 rectal toxicities is often reported to be <5% at PPRT doses of 60-64Gy.1−3 The use of dose escalated PPRT can result in increased late grade 3-4 rectal toxicities.6,7 Multiple studies have showed that the use of hydrogel spacer (HS), inserted in the anterior peri-rectal space, offers the advantage of anterior rectal wall displacement outside the high-dose radiotherapy region, and thus potentially minimises radiation-related rectal toxicities.8 While we would expect the use of HS for PPRT to result in reduced rectal dosimetry, the feasibility of HS insertion following prostatectomy is unclear, and to our knowledge, there has only been three reports in the literature describing the use of HS in the post-radical prostatectomy (RP) setting.9−11 The aim of our current study is to report on our experience in HS insertion for PPRT, and to compare the rectal dosimetry in the pre-HS and post-HS computer tomography (CT) treatment plans.
Study population
This is a retrospective series of prostate cancer patients referred for PPRT following biochemical failure in 2 major radiation oncology services in Australia. This study was approved by the Genesis Care Institutional Review Board (IRB). Because of the retrospective design of our study, the IRB waived the need to obtain informed consent from our patients. Patients presenting with biochemical failure had their macroscopic local recurrence proven with either a prostate specific membrane antigen positron emission tomography (PSMA PET) scan and/or on magnetic resonance imaging (MRI) scan. MRI defined recurrences without a positive PSMA PET scan had their local recurrences proven on biopsy of their prostate bed. These patients with macroscopic local recurrences were suitable for dose escalation to the site of gross tumour and were considered for HS insertion.
This is a study on the first 4 consecutive patients who had HS inserted prior to PPRT. The median age was (65-81 years) and all patients underwent a RP between 1-13 years (mean 8 years) prior to presentation. The initial histopathology confirmed Gleason 7-9, pT2c-T3bN0 adenocarcinoma with negative margins achieved in 3 patients. Prostate specific antigen (PSA) natured at an undetectable level in 3 patients, the PSA at presentation ranged from 0.39 to 7, and all had PSA doubling times of > 6 months. The patient characteristics are detailed in Table 1. All patients had initial staging CT abdomen and pelvis, and whole-body bone scans. A PSMA PET scan was performed in 3 patients and all 4 patients had MRI scans of their pelvis. The MRI scans revealed macroscopic local recurrences in all 4 patients [unifocal recurrence at the right seminal vesicle remnant in 1 patient and vesico-urethral anastomosis (VUA) in 3 patients. The PSMA PET scan confirmed MRI detected local recurrences in 2 patients (Figure 1). The other 2 patients underwent transperineal biopsies of their prostate bed to confirm local recurrence in their VUA. All 4 patients had MRI or CT scans that confirmed a clear fat plane between the local recurrence (prostate bed in 3 patients, right seminal vesicle in 1 patient) and the anterior rectal wall.
|
Patient 1 |
Patient 2 |
Patient 3 |
Patient 4 |
|
|
Age (years) |
65 |
75 |
66 |
81 |
|
Year of RP* |
2009 |
2006 |
2016 |
2004 |
|
Gleason Score |
3+4 |
3+4 |
4+5 |
3+4 |
|
pTNM |
T2cN0M0 |
T2cN0M0 |
T3bN0M0 |
T3aN0M0 |
|
Margin Status |
Negative |
Negative |
Positive |
Negative |
|
Year of presentation |
2017 |
2017 |
2017 |
2017 |
|
PSA# at presentation (ng/ml) |
7.1 |
2.3 |
0.4 |
0.45 |
|
PSADT^ |
>1 year |
>6 months |
>1 year |
>1 year |
|
MRI$ |
24mm R seminal vesicle recurrence |
13mm L VUA |
17mm VUA |
25mm VUA |
|
PSMA PET% |
R seminal vesicle |
L VUA |
No uptake seen |
Not performed |
|
Biopsy |
Not performed |
Not performed |
R prostate bed (&GS 4+3) |
Bilateral prostate bed (&GS 4+4) |
Table 1 Patient characteristics
*RP, radical prostatectomy; #PSA, prostate specific antigen; ^PSADT, PSA doubling time; $MRI, magnetic resonance imaging; %PSMA PET, prostate specific membrane antigen positron emission tomography; &GS, gleason score
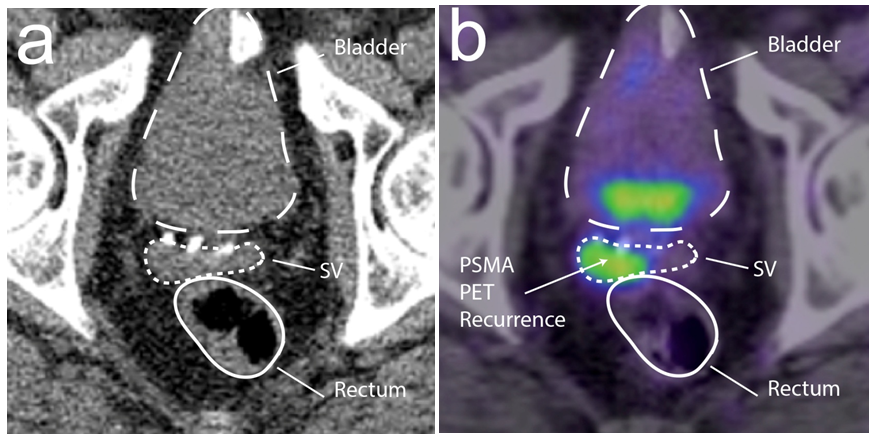
Figure 1 Right seminal vesicle remnant recurrence on (a) computer tomography [CT] and (b) prostate specific membrane antigen positron emission tomography [PSMA PET] scans.
Hydrogel insertion
The non-iodinated HS [ni-HS (SpaceOARä)] or iodinated HS [i-HS (TraceITä)] was injected using a transperineal approach between the local recurrence and rectal wall under transrectal ultrasound guidance with the use of a biopsy template after prior hydrodissection with 10 mls of sterile saline. In addition, at least two gold seed fiducials were inserted into the prostate bed on either side of the VUA for image guided radiotherapy (IGRT). The procedure was performed under general anaesthesia and prophylactic antibiotics were administered.
Radiotherapy planning and treatment
To determine if there were any adverse effects related to the HS insertion, patients were assessed immediately after the procedure and approximately 5-7 days later. After an interval of 5-7 days, patients underwent their CT scan for intensity modulated radiation therapy (IMRT) treatment planning. MRI scans were performed in 2 patients who had ni-HS inserted and they were fused to the planning CT scans to aid with HS volume delineation.
All patients were scanned in the supine position with a full bladder and an empty rectum as per our departmental protocol. The treatment plans were created on the Pinnacle v9.8 (Philips Radiation Oncology Systems, Fitchburg, WI) treatment planning system (TPS). The low dose Clinical target volumes (CTV) encompassed the prostate and seminal vesicle surgical bed at risk of microscopic disease. The high dose CTV encompassed the macroscopic MRI or PMSA defined local recurrence. The CTV to planning target volume (PTV) expansion was 10 mm in all directions. The prescription dose was 67.2 Gy at 1.6 Gy per fraction to the low dose PTV and 75.6 Gy in 1.8 Gy per fraction to the high dose PTV over 42 days, delivered to >95% of the PTV (D95). Rectal volume radiation dose constraint objectives for rV30 (percentage of rectal volume receiving radiation dose in Gy), rV40, rV50, rV60, rV70, rV75 and rV78 were 80%, 65%, 50%, 35%, 20%, 15% and 5%, respectively.
The bladder was contoured from apex to base. The rectum was contoured as a whole solid structure beginning at 1.0cm above the most superior level of the PTV to the anorectal junction as per departmental protocol. In order to determine the effect of the HS for each patient, two treatment plans were created from the baseline pre-HS CT and the post-HS CT+/-MRI scans (Figure 2). Pre-HS treatment plans were created from either CT simulation scans performed prior to the HS insertion or reconstructed from diagnostic CT scans performed during the patient’s initial workup. The degree of separation achieved between the anterior rectal wall and the posterior edge of prostate bed was quantified for the pre-HS and post-HS treatment plans. The rV30, rV40, rV50, rV60, rV70, rV75 and rV78 were compared. The low dose (67.2Gy) and high dose (75.6Gy) PTV volumes, rectal volume and D95 for PTV67.2 and PTV75.6 were also compared to ensure consistency between the pre-HS and post-HS treatment plans.
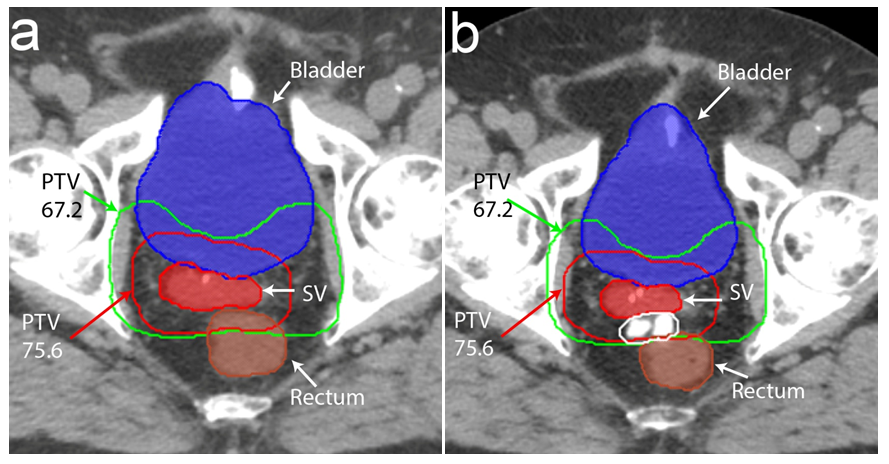
Figure 2 Pre-hydrogel spacer (HS) and post-HS treatment planning scans showing bladder (solid blue), rectum (solid brown), seminal vesicle recurrence (solid red), low dose PTV (PTV67.2) in green and high dose PTV (PTV75.6) in red. The iodinated HS is hyperintense on computer tomography (delineated by white line) and displaces the rectum posteriorly. The low and high dose PTV transects the rectum in the pre-HS treatment planning scan but is situated on the anterior surface of the rectum post-HS.
None of the 4 patients reported bleeding or infection following the insertion of HS. There were no allergic reactions or reports of urinary retention, tenesmus or rectal ulceration following the insertion of HS. We were able to evaluate the pre-HS and post-HS plans and found them comparable, with no significant differences between the PTV low and high dose volumes, the rectal volumes and the PTV low dose and high dose volume dosimetry (Table 2). The distance between the rectal wall and local recurrence was increased from immediate vicinity to a mean of 12mm (11-16.5mm) after HS insertion. The HS did not have an impact on rectal volume radiation dose endpoints until at least 60 Gy with a more significant influence at higher doses (Table 3). The difference between pre-HS vs post-HS plans were 20.3% for rV60; 51% for V70; 92.9% for V75 and 100% for V78. The median follow up post IMRT is 16 months (range 14-16 months). Two patients have achieved undetectable PSA levels while the other two have experienced continuing drop in their PSA level to 0.4 ng/ml and 0.2 ng/ml. Only 1 patient developed common terminology criteria for adverse events (CTCAE) grade 1 acute gastrointestinal (GI) toxicity. So far, there has been no late GI toxicity reported.
|
Structure Volumes |
Pre-HS*(mean + SD^) |
Post-HS*(mean + SD^) |
P value |
|
+PTV67.2 (low-dose) Volumes (cc) |
365.8 + 35 |
355 + 18.1 |
P=0.35 |
|
+PTV75.6 (high-dose) Volumes (cc) |
100.3 + 14.3 |
99.8 + 13.7 |
P=0.9 |
|
+PTV67.2 (low-dose dosimetry) D95# |
97.8 + 2 |
98.1 + 0.5 |
P=0.78 |
|
+PTV75.6 (high-dose dosimetry) D95# |
97.1 + 1.3 |
97.5 + 0.6 |
P=0.47 |
|
Rectum (cc) |
70.5 + 4.8 |
62.8 + 6.5 |
P=0.09 |
Table 2 Pre-HS* and post-HS* structure volumes and PTV+ D95#
*HS, hydrogel spacer; ^SD, standard deviation; +PTV, planning target volume; #D95, prescription dose delivered to 95% of planning target volume
|
Rectal Dose Endpoints |
Pre-HS* Plan (% volume) |
Post-HS* plan (% volume) |
% Difference |
P Value |
|
mean + SD^ |
mean + SD^ |
|||
|
+rV30 |
79.9 +/-9.4 |
70.3 +/-8.2 |
11.9 |
P=0.26 |
|
+rV40 |
65.9 +/-6.3 |
58 +/- 9.6 |
12 |
P=0.3 |
|
+rV50 |
56.1 +/-4.4 |
47.7 +/-10 |
14.9 |
P=0.26 |
|
+rV60 |
45.4 +/- 2.1 |
36.1 +/-9.7 |
20.3 |
P=0.17 |
|
+rV70 |
21.1 +/-4.3 |
10.4 +/-6.3 |
51 |
P=0.06 |
|
+rV75 |
6.0 +/-1.9 |
0.4 |
92.9 |
P=0.01 |
|
+rV78 |
0.1 |
0 |
100 |
P=0.28 |
Table 3 Pre-HS* and post-HS* rectal volume radiation dose endpoints
*HS, hydrogel spacer; ^SD Standard deviation; +rV, rectal volume receiving radiation dose in Gray
We report the feasibility of HS for PPRT, with significant reduction in the rectal dosimetry. The insertion of HS in our series of 4 patients resulted in a median of 12mm (11-16.5mm) posterior displacement of rectal wall, and 51% reduction in rV70. Apart from two case reports by Pinkawa et al.9 and Arcangeli et al.10 and a series of 32 patients reported by Yeh et al.11 in abstract, this is only the fourth and second largest series on the use of HS with PPRT. Several studies have shown the oncological benefits of dose escalation in PPRT.5,12,13 In a single institution study in US, Valicenti et al. reported that the 3-year biochemical relapse free survival (bRFS) was better with PPRT dose of >61.2Gy in the adjuvant setting (90% vs. 64%), and PPRT dose of >64.8Gy in the salvage setting (52% vs 18%).12 Given the evidence for higher doses in PPRT, multiple national guidelines, including the American Society for Radiation Oncology/ American Urological Association guidelines,14 the German Prostate Cancer Guidelines,15 and the Australian and New Zealand Radiation Oncology Genitourinary Group guidelines,16 have all recommended RT doses in the range of at least 64-66 Gy for salvage PPRT versus 60Gy for adjuvant PPRT. However, there is emerging data for even higher doses, with Cozzarini et al. reporting improved 5-year bRFS (83% vs. 71%), and disease free survival (94% vs. 88%), for dose escalated PPRT to >70.2Gy compared to <70.2Gy.13 In a systematic review, King et al. reported that the dose response fits a sigmoidal curve for PPRT and parallels that for definitive radiation therapy (RT) for localised disease, with a dose of 70Gy achieving 58.6% bRFS vs 38.5% for 60Gy.17 The expected proportional gain in bRFS is 2% per incremental Gy. The ongoing phase 3 Swiss Group for Clinical Research 09/10 trial will randomise patients without macroscopic disease to either 64Gy or 70Gy and will help provide further insight into the value of dose escalation.18 In the setting of macroscopic disease, our departmental protocol encourages the use of dose escalated PPRT as recommended by Ost et al.6 where possible.
However, given the proximity of rectum to the prostatic bed, dose escalation for PPRT can be associated with increased rectal toxicities. Goenka et al. reported their late grade 2/3 genitourinary (GU) and GI toxicities for their IMRT cohort receiving >70Gy at 16.8% and 1.9%.7 Ost et al. delivered far higher PPRT doses with a median of 76Gy and reported late grade 2/3 GU and GI toxicities at 22% and 8%.6 Ohri et al. reported increased late gastrointestinal toxicity in PPRT by 1.2% per Gray.19 The use of HS to increase the spatial separation of rectal wall from the high dose region is therefore an appealing approach to reduce the rectal dosimetry, while allowing for dose-escalation to the target volume (gross tumor). The use of HS has been well established in the setting of intact prostate RT with the randomised SpaceOAR study by Mariados et al. demonstrating a reduction in rV70 from 12.4% to 3.3%, a relative reduction of 73.3% in favour of the HS patients.20 This translated to a reduction in late rectal toxicity from 9.2% to 2%, with no late grade 2 or greater toxicity seen at 3 years.21 In addition, bowel quality of life score (QOL) benefits as measured by the Expanded Prostate Cancer Index Composite tool has consistently favoured the HS patients from 6 months onward, with the difference at 3 years >5 points, meeting the threshold for a minimally important difference. This has also been confirmed by Pinkawa et al. with bowel QOL improvement continuing to 5 years for the HS cohorts.22
However, insertion of HS for PPRT can be challenging, given that the plane for HS insertion is less well-defined following prostatectomy. This is in contrast to HS insertion in the intact prostate RT setting, whereby there is a well-demarcated prostatic capsule and the HS is inserted into the potential space immediately posterior to the Denonvillier’s fascia. One of the concerns that need to be taken into consideration during insertion of HS for PPRT is the possibility of posterior displacement of cancer cells. This may lead to under-treatment of the cancer cells, posterior to the HS, and may therefore compromise on the oncological control. A prerequisite for HS insertion was successful hydrodissection between the local recurrence and rectal wall, as this would have been impossible in the setting of rectal wall cancer infiltration. Hence, it is important that we carefully select patients in who there is convincing macroscopic local recurrence, and where there is no ambiguity as to whether there is involvement of the anterior rectal wall.9
Reassuringly, the anterior rectal wall has not been shown to be common site of local recurrence following RP,23−28 which suggest that there is potential role for HS in most patients with macroscopic local recurrence. Earlier studies using transrectal ultrasound guided biopsies to identify the site of biochemical failure following RP showed that the peri-anastomotic site was the most common site of recurrence, with incidence in the range of 60%.23−25 MRI studies among patients with local recurrence also showed that large majority of recurrence sites were peri-anastomotic, retro-vesicle, and seminal vesicle.26−28
It is important to acknowledge several limitations of this study. This is a retrospective study, based on the experience of a single urologist and radiation oncologist in a highly-selected group of patients. We do acknowledge that this is our early experience with HS insertion for PPRT. Pinkawa et al has previously demonstrated a learning curve in the insertion of HS in the intact prostate.29 In this study, the first cohort of 32 consecutive patients had increased mean distance between prostate and anterior rectal wall (1.5cm vs. 1.1cm), and significantly lower rectal V70Gy (6% vs. 2%) compared with the second cohort of 32 patients. With increase experience, we would also expect to achieve better and more symmetrical HS insertion and better rectal dosimetry in the PPRT setting.
In conclusion, we report the largest series on the feasibility of HS insertion for PPRT, with significant reduction in the high dose rectal volume dosimetry, and hence allows for safer dose escalation to the gross tumor volume (local macroscopic recurrence). The use of HS was found to be effective as a spacing agent in this small series of post RP patients.
We wish to thank Lee Tripcony for his statistical assistance.
Nil.

©2019 Chao, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.