International Journal of
eISSN: 2574-8084


Research Article Volume 5 Issue 4
1Department of Surgery, Federal University of São João del-Rei (FM/UFSJ), Brazil
2Department of Surgery, Federal University of Minas Gerais (FM/UFMG) and Felício Rocho Hospital, Brazil
3Felício Rocho Hospital, Brazil
4Ecoar Clinic, Brazil
5Mario Pena Foundation, Brazil
6Department of Surgery, FM/UFSJ, Brazil
Correspondence: Fábio Henrique de Oliveira, Department of Surgery, Medical School, Federal University of São João del-Rei (FM/UFSJ), Brazil, Tel +55 (37) 999643793
Received: July 05, 2018 | Published: August 8, 2018
Citation: Oliveira FH, Filho AL, Queiroz FL, et al. Magnetic resonance imaging is effective in assessing tumour regression after neoadjuvancy in rectal adenocarcinoma. Int J Radiol Radiat Ther. 2018;5(4):254-258. DOI: 10.15406/ijrrt.2018.05.00173
Introduction: Rectal cancer (RC) is a tumor commonly diagnosed in developing countries, accounting to the considerable rates of morbi-mortality. The neoadjuvant therapy (NT) with radiotherapy and chemotherapy plays a leading role in the management of this neoplasm, according to oncological results and the preservation of anal function. Magnetic resonance imaging (MRI) is one of the main tools for the evaluation of NTresults in rectal cancer in order to manage the patient’s therapy.
Objectives: To compare the results of the MRI performed right after NTwith histopathologic findings of surgical specimens in patients with RC whom underwent radical surgery.
Methods: This retrospective study of 31 patients with extraperitoneal RC Whom underwent radical surgery with a total mesorectal excision (TME) and with the preservation of sphincter after performing an NT. The selected patients underwent an pelvis MRI with an emphasis on the rectum, before and after performing an NT. The initial stage of the disease was detected on the first MRI, and the second MRI (post-NT) was responsible for determining the tumor’s regression grade (TRG) and downstaging disease. This TRG was compared with the estimated regression obtained by histopathology of the specimen based on Dworak-TRG classification and TNM classification and trying to analyze the degree of agreement of the two methods and the occurrence of downstaging.
Results: Most patients presented a downstaging of (74.4%) of thedisease after NT, when compared to the initial stage. Six patients (19.4%) achieved a complete pathological response. An MRI had a sensitivity of 83.3% and specificity of 73.7%, with Positive Predictive Value PPV=66.7%, Negative Predictive Value NPV=87.5% and an accuracy of 0.774 in estimating the TRG compared to histopathology. There was no correlation between patient characteristics and pathological findings and the results of TRG.
Conclusion: An MRI of the pelvis with an emphasis on the rectum has a high accuracy rate when evaluating patients with RC submitted to NTwith radiotherapy and quimioterapy compared with histopathology and should be used routinely in clinical practice.
Keywords:rectal cancer, magnetic resonance imaging, tumor regression grade
Colorectal adenocarcinoma is the third most common malignant neoplasia and the third leading cause of death from cancer in men and women in the United States. Current data show that the incidence of colorectal adenocarcinoma is decreasing in developed countries but increasing in developing countries.1 The 2018 estimates of the Brazilian National Cancer Institute (Instituto Nacional do Câncer–INCA) were 17,380 new cases in men and 18,980 in women, making colorectal adenocarcinoma the third most common neoplasia in men and the second most common in women in Brazil.2 In the past 15 years, rectal cancer management has evolved in several aspects. Specifically, a better understanding of the natural history of the disease, more precise radiological staging, multimodal therapeutic intervention, refined surgical techniques, and more detailed histopathological reports may have positively influenced patient survival. In this context, multidisciplinary management of colorectal cancer plays an important role and requires the coordinated teamwork of colorectal surgeons, oncologists, radiologists, and radiotherapists.3 Total mesorectal excision is still the basis of treatment in rectal cancer. However, neoadjuvant therapy and more conservative practices have been adopted in cases of clinical/pathological responses to radiochemotherapy.4 Radiological evaluation of the response is of paramount importance for the selection of patients eligible for alternative treatment strategies, including ‘watch-and-wait’. Diffusion-weighted imaging is already being used routinely in the evaluation of the pathological response of rectal tumour patients submitted to neoadjuvant therapy. Some researchers have tried to estimate the tumour regression grade (TRG) using magnetic resonance imaging, as has been described for post-radiochemotherapy pathological evaluation, thus rendering it a valuable instrument.
Considering the good results obtained with multimodal therapy in extraperitoneal rectal cancer, the evaluation of the pathological response post-neoadjuvant therapy must be considered as a factor for safe indication, both for the conservative option, in which the organ is preserved, and for radical surgical resection, influencing the choice between sphincter-preserving surgery and abdominoperineal excision. A precise evaluation, by comparing the results of post-neoadjuvant therapy magnetic resonance imaging with those obtained from histopathology, could further substantiate the indication of this imaging method in post-neoadjuvant therapy tumour staging. Thus, the present study sought to assess the agreement rate of post-neoadjuvant therapy tumour regression grading via magnetic resonance imaging vs. histopathological exam in patients with extraperitoneal rectal cancer.
The present work is a non-concurrent prospective study on 31 patients with middle and lower rectal cancer subjected to total mesorectal excision with preservation of the anal sphincter after neoadjuvant radiochemotherapy. All patients were operated by the same surgical team of the Coloproctology Clinic at Felício Rocho Hospital in Belo Horizonte, Minas Gerais, Brazil, from April 2008 to September 2015. Patients were subjected to magnetic resonance imaging of the pelvis with emphasis on the rectum before and after neoadjuvant therapy. Patients aged 18 years and above, who were diagnosed with middle and lower rectal adenocarcinoma stages II, III, and IV by means of digital rectal exam, endoscopy, and biopsy, and who agreed to participate were included in the study. All participants signed an informed consent form after being acquainted with the objectives of the study and clarifying any doubts. The following inclusion criteria were used: patients who completed neoadjuvant therapy, attending all scheduled sessions of radiotherapy and chemotherapy cycles; patients submitted to magnetic resonance imaging of the pelvis with emphasis on the rectum before and after neoadjuvant therapy, and with both exams available for evaluation; and patients undergoing surgery 6 to 8 weeks after the completion of neoadjuvant therapy. All patients were subjected to neoadjuvant rectal cancer therapy consisting of a total radiation dose of 54Gy for 5 weeks and concomitant chemotherapy consisting of two cycles of intravenous bolus injections of 5-fluorouracil, 400mg/m2/d, and leucovorin, 20mg/m2/d, for three days in the first and fifth weeks of radiotherapy.
Magnetic resonance images were evaluated by a radiologist experienced in studying the pelvis with emphasis on the rectum, who had no access to the pathological exam of the resected specimen. All of the initial exams performed prior to neoadjuvant therapy were analysed thereafter, which involved completing a specific form with the tumour-node-metastasis (TNM) staging system. After a minimum interval of one week, all post-neoadjuvant therapy exams were evaluated by completing a similar form, differing only in the classification of T3 tumours. Tumour response was characterised as the substitution of high signal intensity by fibrosis, which appears as low signal intensity (dark stroma), or by high signal intensity mucin pools. Tumour regression analysis via magnetic resonance imaging was performed using a tumour regression grading system (mrTRG) similar to that adopted by Dworak in the evaluation of pathological regression (dTRG), as shown Table 1.5 For the histological exam, samples were processed according to the usual histological routines, including dehydration in alcohol and xylol followed by embedding in paraffin. Paraffin blocks were cut into 3- to 4-μm thick histological slices, and slides were stained with haematoxylin and eosin (HE) for examination under a light microscope. Each case was examined twice by the same examiner. Samples were classified by the traditional post-treatment staging system (yTN) and the dTRG system, as shown in Table 2 & Figure 1.5 Histo-pathological staging post-NT will be called ypT. Furthermore, a simplified pathological classification was defined, comprising two new groups: those with favourable and those with unfavourable responses. Favourable pathological findings were defined as ypT stages 0, 1, and 2 or dTRG stages 3 and 4. Unfavourable findings were determined as ypT stages 3 and 4 or dTRG stages 0, 1, and 2. This new grouping was possible because studies have shown that well-responding patients exhibit a similar behavior.6
Original dTRG |
Modified dTRG |
mrTRG |
4 |
1 |
1 |
3 |
2 |
2 |
2 |
3 |
3 |
1 |
4 |
4 |
0 |
5 |
5 |
Table 1 Modified dworak classifications (dTRG)
mrTRG |
|
1 |
6(19.4) |
2 |
9(29.0) |
3 |
10(32.3) |
4 |
6(9.4) |
5 |
0(0.0) |
dTRG |
|
0 |
4(12.9) |
1 |
6(19.4) |
2 |
9(29.0) |
3 |
6(19.4) |
4 |
6(19.4) |
Table 2 Tumour regression grading using magnetic resonance (mrTRG) and histopathology according to dworak (dTRG) (n=31)
The dTRG defines full tumour regression as a score 4, and partial responses are scored in a decreasing manner, until a score of 0 (zero), which refers to the absence of regression. In contrast, the mrTRG system analogously considers the same result but with inverse scoring, i.e., a pathologic complete response (pCR) is a score of 1, and partial responses receive increasing scores up to 5, which represents the absence of regression. For an adequate comparison between the variables related to dTRG and mrTRG and to aid the statistical analysis, the scores of the Dworak classification were modified. Specifically, a score of 1 was attributed to pCR, whereas the absence of a pathologic response received score of 5, and in-between stages followed the numerical order, as shown in Table 3. This modification was needed to assess tumour regression numerically. Patients were divided into two sub-groups according to pathologic and radiological responses via dTRG and mrTRG, respectively. Those with dTRG 4 and dTRG 3, mrTRG 1, and mrTRG 2 were considered good responders, whereas those exhibiting dTRG 2, dTRG 1, and dTRG 0 and mrTRG 3, mrTRG 4, and mrTRG 5 were considered poor responders. The present study was submitted to and approved by the Research Ethics Committee of the Felício Rocho Hospital via the Plataforma Brasil - Sisnep. The project is registered under CAE number 11051212.4.0000.5125.
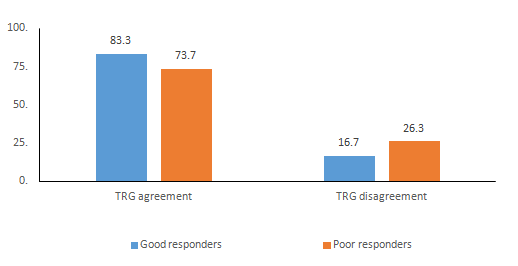
Figure 1 Analysis of agreement between dTRG and mrTRG among good and poor responders to neoadjuvant therapy.
Statistical analysis
A descriptive analysis was performed by calculating the means ± standard deviations for quantitative variables and absolute frequencies and percentages for categorical or qualitative variables. The agreement between the tumour regression analysis methods, i.e., via pathological exam (dTRG) or magnetic resonance imaging (mrTRG), was assessed using the Kappa test and the diagnostic test measures sensitivity, specificity, positive predictive value (PPV), and negative predictive value (NPV). The association between the studied variables and good tumour regression grades according to dTRG and mrTRG was assessed using multivariate logistic regression, after univariate regression of all variables with the variable response. An additional analysis was performed to assess variables that could positively influence the response to treatment, i.e., that possibly contributed to tumour regression, using the Pearson’s Chi-squared test (more than 20% of the expected value between 1 and 5), and univariate logistic regression. The level of significance was set at 0.05. Statistical analyses were performed with IBM® SPSS® Statistics version 20.0, October/2012.
There was a discrete predominance of women among the participants (54.8%). The mean patient age was 66.2±11.9 years, and the mean tumour distance from the anal verge was 7.4±2.4cm. In most patients (74.4%), tumours were located 5 to 12cm from the anal verge, which corresponds to the middle rectum, whereas seven patients (22.6%) exhibited lesions in the lower rectum, up to 5cm from the anal verge. Tumour staging (T) via magnetic resonance imaging assessed prior to neoadjuvant therapy revealed that most lesions (64.5%) were T3 tumours, followed by T2 tumours (32.3%) and a lower percentage of T4 tumours (3.2%). The distribution of pre-neoadjuvant treatment T staging via magnetic resonance imaging is detailed in Table 2. Regarding affected lymph nodes, 17 patients (54.8%) exhibited no invasion (N0). The following stages were classified using magnetic resonance imaging: I (12.9%), IIA (41.9%), IIIA (19.4%), IIIB (22.6%), and IIIC (3.2%). A pCR to neoadjuvant radiochemotherapy was observed in six patients (19.4%). Concerning tumour regression grading assessed with magnetic resonance imaging, 19.4% of the patients exhibited pCR (mrTRG 1); 29.9% exhibited dense hypointense fibrosis with minimal residual tumour (mrTRG 2); 32.3% exhibited mixed areas of fibrosis, mucin, and intermediate signal representing residual tumour, with predominant fibrosis (mrTRG 3); 9.4% exhibited minimal fibrosis, mucinous degeneration, and tumour predominance (mrTRG 4); and 0% exhibited absence of response (mrTRG 5). Concerning tumour regression grading via histological exam according to Dworak, 19.4% of the patients exhibited pCR (dTRG 4); 19.4% exhibited a minimal amount of tumour cells (dTRG 3); 29% exhibited predominant areas of fibrosis, but with tumoural foci (dTRG 2); 19.4% exhibited minimal fibrosis with tumour predominance (dTRG 1); and 12.9% exhibited no tumour regression (dTRG 0) (Table 2). The results of the Kappa test, which assessed the agreement between the mrTRG and dTRG methods, revealed a moderate agreement of 0.545 (95% CI), with a standard error (SE) of 0.148. Considering the histopathological analysis as the gold-standard for the determination of tumour regression, magnetic resonance imaging exhibited 83.3% sensitivity and 73.7% specificity, with PPV=66.7%, NPV=87.5%, and an accuracy of 0.774 (Table 3) (Table 4).
mrTRG |
dTRG |
Kappa SE (Kappa) 95 % CI Kappa |
|
Good responders |
Poor responders |
||
Good responders |
10 |
5 |
0.545 SE = 0.148 |
Poor responders |
2 |
14 |
|
Table 3 Analysis of agreement between mrTRG and dTRG
CI, confidence interval; SE, standard error
Sensitivity |
Specificity |
PPV |
NPV |
Accuracy |
0.833 |
0.737 |
0.667 |
0.875 |
0.774 |
0.516;0.979 |
0.488;0.909 |
0.384;0.882 |
0.617;0.985 |
|
Table 4 Analysis of agreement between mrTRG and dTRG
PPV, positive predictive value; NPV, negative predictive value
Neoadjuvant radiochemotherapy followed by radical surgical resection currently constitute the standardised therapeutic approach in the treatment of locally advanced extraperitoneal rectal cancer. This multimodal therapy aims to reduce recurrence and to increase chances of sphincter preservation. Lately, the above multidisciplinary approach has allowed for the preservation of the organ in some selected cases.7 According to the Mercury study, the evaluation of tumour regression by magnetic resonance imaging is an important marker of survival, which can determine good and poor responders and can also influence therapeutic modalities.8,9 Re-staging after neoadjuvant therapy is necessary because it allows for the evaluation of tumour response to radiochemotherapy, which might help in the choice of the surgical strategy to be adopted. In the histopathological exam of the resected specimen, the substitution of tumour cells by fibrous tissue, either completely or partially, is assessed, thus conferring an important impact on post-operative results.10 The present study assessed the capacity of magnetic resonance imaging of the rectum to predict tumour response to neoadjuvant treatment in patients with extraperitoneal rectal cancer. For this evaluation, mrTRG was compared with the histopathological method (dTRG), which was considered as the gold-standard in this context. Patients were divided into two subgroups, good and poor responders, based on the similar prognosis between those who exhibited complete or nearly complete tumour regression.11 The agreement among the good responders was moderate, with 83.3% sensitivity and a Kappa of 0.545. This finding indicates that patients with rectal tumours who exhibit a favourable response to neoadjuvant therapy can be re-staged by means of magnetic resonance imaging with good accuracy.
Currently, minimally invasive or even non-surgical technical modalities have been suggested for the treatment of rectal cancer, aiming to preserve not only sphincter function but also the sphincter itself and its functional conditions. Magnetic resonance imaging is an effective and safe method for the re-evaluation of good responders, who, in theory, could be eligible for organ-sparing treatment. A recent study has shown that the tumour volume reduction rate associated with regression according to magnetic resonance imaging are the main predictors of pCR.12 Similarly, patients with an unfavourable response, i.e., those who exhibited no satisfactory tumour regression, exhibited 73.7% specificity. This value indicates that magnetic resonance imaging has moderate agreement in determining those considered as poor responders. In practical terms, re-staging of poor responders has no influence on the decision regarding treatment strategy choices, as those patients already exhibit a significant residual lesion in the digital rectal exam and in the proctoscopy during the post-radiochemotherapy clinical evaluation. Thus, these patients are evidently eligible for conventional radical surgical resection.
The accuracy of magnetic resonance imaging in determining tumour regression was 77.4%, which can be considered satisfactory. The values found in this study are in agreement with those published in the literature. In an analysis of 78 patients, Patel et al. found moderate agreement between mrTRG and dTRG, with a Kappa value of 0.55. In contrast, prospective multicentre studies have found higher agreement rates than that obtained by the Mercury group, with a Kappa value of 0.65.13 According to Habr-Gama et al.,6 more than 50% of all patients undergoing neoadjuvant therapy can exhibit a clinical complete response (cCR), which is retained for more than 12 months, with failure rates of approximately 17%.14 These values are considerable when the aim of rectal cancer management is a non-operative approach, and re-staging using imaging plays a fundamental role in this strategy. The comparison between re-staging using magnetic resonance imaging vs. histopathology can pose problems of agreement due to the occurrence of overstaging. It has been shown that 24% of T and 36% of N stages were overestimated in magnetic resonance imaging after neoadjuvant therapy. These findings are essentially related to two histopathological factors, i.e., the marked presence of fibrosis in the rectal wall and peritumoural inflammatory cell infiltration.15 In the present study, good responders exhibited tumour regression rates of 19.4% for dTRG 4 and dTRG 3, each. Rodel et al.,13 found the following results in a sample of 385 patients: dTRG 4=10.4% and dTRG 3=52.2%. Of note, the results are similar when grade three and four responses are grouped together. Tumour regression rate and oncological results are known to be strongly correlated. Patients staged dTRG 4 or dTRG 3 exhibit better control of lymph node disease, better local control of the disease, and lower chances of developing distant metastases.13
To date, magnetic resonance imaging is the main tool for rectal cancer staging, definitely reinforcing the indication for neoadjuvant therapy based on the findings of this imaging exam, associated with the proctologic examination. Currently, T3-4/N+ tumours are considered to be those with the best indication. However, some researchers estimate that approximately 15-30% of patients are being overtreated, i.e., that they could undergo isolated surgery instead, with no need to be exposed to the possible adverse effects of neoadjuvant radiochemotherapy.3 For this reason, other parameters for the indication of neoadjuvant therapy have been investigated. Among the patients of the present study, 19.4% exhibited pCR. In these cases, no tumour cells were found in the microscopic evaluation of the resected specimen. This finding is in agreement with the data reported in the literature. A recent study shows that the majority of patients undergoing neoadjuvant radiochemotherapy experience downstaging and that 15-27% of the patients exhibit pCR.16 In 2004, Dr Habr-Gama and her group, pioneers in the ‘Watch-and-Wait’ protocol, showed that 26.8% of the 265 patients undergoing neoadjuvant radiochemotherapy alone developed cCR, with 4% exhibiting distant metastases and 2.8% developing local recurrence, the latter being submitted to salvage surgery. More recently, and using a more extensive chemotherapy protocol, the same group achieved a cCR in 68% of the studied patients. Of these, 6% exhibited distant metastases, and 25% developed local recurrence and were thus eligible for salvage radical surgery.7
In view of this context, and aiming to preserve the rectum, follow-up must be thorough, with clinical, endoscopic and imaging methods adequately accurate for the evaluation of the rectal wall and adjacent structures, especially lymph nodes. Among the imaging methods, magnetic resonance imaging is efficient and safe and should be performed 12 weeks after the end of neoadjuvant therapy and thereafter in six-month intervals during the first two years of follow-up.8 TRG has also been evaluated from the standpoint of an indication of adjuvant therapy and has not been shown to be a good indicator of chemotherapy after resection.17 Bohlok et al.,18 in a study with 74 patients demonstrated that it was not safe to have TRG with predictor of adjuvant treatment.18 Preventing a rectal cancer patient from being subjected to surgical resection is not yet an option, except in scientific investigation protocols. In turn, neoadjuvant therapy protocols and the ideal form of follow-up are not yet in agreement. In a recent study, Nahas et al.,10 showed, in contrast to most studies, that the clinical and radiological criteria for the identification of pCR exhibit low sensitivity (18.2%), with only 5% of the patients exhibiting pCR.10
More recent studies still show that an MRI has a moderate and even low degrees of accuracy, even when working with a larger number of patients, as demonstrated by JK Jang et al.,17 whom was in his article had a sampled 439 patients.17 Another current limitation is the fact that there is no specific protocol for the determination of tumour regression rates with either magnetic resonance imaging or histopathological analysis. This lack motivated the development of a specific questionnaire for the collection of these data, which were obtained retrospectively. Thus, we show here that magnetic resonance imaging is an important tool in predicting response to treatment in a context where the beneficial effects of neoadjuvant therapy are evaluated with respect to pCR, aiming at preserving the rectum. We further show that the tumour regression rate in patients with favourable responses exhibits considerable agreement with histopathology. Thus, magnetic resonance imaging is sufficiently accurate in re-staging rectal cancer patients after neoadjuvant radiochemotherapy, and its use should be incorporated into routine clinical practice.
Funding
There was no funding related to this study
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. Informed consent Informed consent was waived by our institution’s research and development department.
None
The author declares no conflicts of interest.

©2018 Oliveira, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.