International Journal of
eISSN: 2574-8084


Case Series Volume 7 Issue 3
1St Vincent’s Hospital, Australia
2GenesisCare Radiation Oncology, Australia
3Eastern Suburbs Dermatology, Australia
4The Skin Hospital, Australia
5Blacktown Dermatology, Australia
Correspondence: Gerald B Fogarty, Director, Department of Radiation Oncology, Mater Hospital, 25 Rocklands Rd, Crows Nest NSW 2065, Australia, Tel +61 2 94588050
Received: May 11, 2020 | Published: May 20, 2020
Citation: Butcher LM, Fogarty GB, Sinclair S, et al. Less is more when treating the nasal ala with superficial radiotherapy. Int J Radiol Radiat Ther. 2020;7(3):66-69. DOI: 10.15406/ijrrt.2020.07.00267
Skin cancer is the most common malignancy, is increasing in incidence, and occurs most commonly on the head and neck. Cancers of the nasal ala pose therapeutic challenges given the cosmetic and functional importance. Both surgery and radiotherapy (RT) have similar oncological outcomes. RT is tissue-conserving and may have an advantage in cosmetic and functional outcomes, but more comparative trials are needed. RT needs to be delivered well to avoid late effects such as skin atrophy, fibrosis and telangiectasia, which may increase with higher dose per fraction. We describe three cases of self-reported thinning of the nasal ala following definitive mildly hypofractionated superficial radiotherapy (SXRT) of 2.5 Gy per fraction. SXRT to skin cancers of the nasal alar with standard fractionation of at most 2 Gy per fraction may be important in ensuring excellent cosmetic outcomes and patient satisfaction.
Keywords: case report, radiotherapy, radiotherapy dosage, dose fractionation, skin cancer, nasal ala, external nose, Australia
ASCRT, advanced split course radiation therapy; BCC, basal cell carcinoma; cm, centimetre; ESFC, extensive skin field cancerisation; Gy, Gray; GTV, gross tumour volume; HVL, half-value layer; Kv, kilovoltage; mm, millimetre; NMSC, non-melanoma skin cancer; RT, radiotherapy; SCC, squamous cell carcinoma; SXRT, superficial radiotherapy
Australia has the highest rate of skin cancer in the world.1 Melanoma and non-melanoma skin cancers (NMSC), such as basal cell carcinoma (BCC) and squamous cell carcinoma (SCC), account for the largest number of cancers diagnosed annually in Australia. Cancer of the nasal ala is relatively common and a challenge to treat due to unique anatomical and functional considerations.2,3 Focusing on nasal function and cosmesis is important to ensure a patient’s quality of life. The complexity of nasal anatomy means that skin cancer treatment at this site becomes more challenging. The skin overlying the alar cartilages and nasal tip consists of fibrofatty tissue which is more firmly adherent to the subjacent parts, compared to the thinner and more mobile skin overlying the nasal dorsum.4 Muscles on the nose include the nasalis, which consists of two parts, the compressor nasi and the alar nasalis. The former functions to contract the nostrils and narrow the vestibules, and the latter assists in dilatation of the nares. The internal and external nasal valves, which are created by a three-dimensional construct including the nasal ala, play a crucial role in airway resistance. The nasal alar blood supply courses between the external skin surface and the internal mucosal surface. These are separated by millimeters, making surgery challenging.
Surgery and radiotherapy (RT) to the nasal ala for skin cancer can affect function and cosmesis.5 Surgery involves tissue loss which may lead to impaired function and cosmesis (Figure 1). Severe cases, especially recurrent disease in immunosuppressed patients, may lead to nasectomy (Figure 2). Fractionated RT can treat a mixed population of tumour and normal cells. It can eradicate tumour and conserve normal tissue due to differences in the radiation repair capacity between the fractions of the different cell populations.6‒8 Standard RT fractionation that results in almost complete conservation is 1.8–2 Gray (Gy) per fraction. At this dose, late fibrosis is minimal compared with higher dose per fraction.9 More fractionation requires more visits to a RT department. This is less practical, especially amongst patients with mobility issues, and there is constant pressure to hypofractionate, using fraction sizes over 2 Gy, to reduce the cost and inconvenience of RT. If RT is given this way the risk is that late effects, dominated by skin atrophy and fibrosis, may cause similar problems to surgery (Figure 3). We present three cases of self-reported atrophy of the nasal ala following definitive RT for skin cancer. All were treated on an Xstrahl® machine (Xstrahl Ltd, Camberley, Surrey, UK).
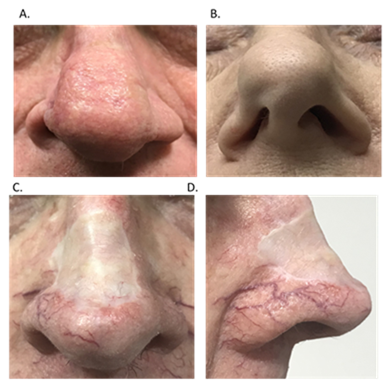
Figure 1 Surgery involves tissue loss which may lead to impaired function and cosmesis. (A) Surgery with primary closure for skin cancer of the right nasal ala has led to asymmetry of the nose. (B) Surgery for skin cancer has led to a flail right nasal ala, also giving asymmetry of the nose. (C) Anterior view of split skin graft on nose following removal of skin cancer with impacts on function and cosmesis. (D) Lateral view of same split skin graft on nose following removal of skin cancer.
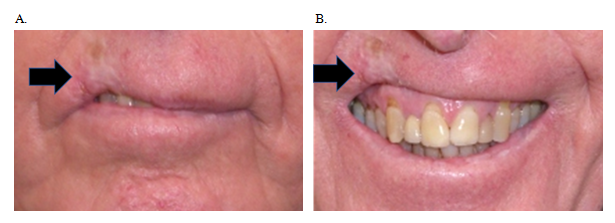
Figure 3 An SCC on the right upper lip was treated successfully with hypofractionated RT using superficial x-ray radiation therapy (SXRT) of 36 Gy in 6 fractions given twice a week. RT late effects dominated by fibrosis have led to a defective smile as a result of telangiectasia, cicatrisation, atrophy and hypopigmentation (black arrow). (A) With mouth neutral. (B) Smiling – note retraction of the tissue caused by late effects of RT.
Case 1
A 71-year-old female with no comorbidities presented with a previously untreated, biopsy-proven 1.4 mm thick nodular BCC on the right nasal ala groove. The lesion measured 15 mm in diameter (Figure 4A). She was treated on with SXRT 100 kilovoltage (Kv), with half-value layer (HVL) of 3.95 millimeters (mm), and a source-to-surface distance of 30 centimeters (cm), dosed to the surface and collimated with a three cm cone. Her nose was packed with a water equivalent bolus prior to therapy with an internal nose shield. She was treated over 32 days. She had 22 Gy in 10 fractions and was then treated with an increased fraction size using a further 30 Gy in 12 fractions that is 2.5 Gy per fraction, to a total dose of 52 Gy. She had moist desquamation at the end of treatment (Figure 4B). Seven months post treatment she reported her right nasal ala to be thinned compared to the left (Figure 4C&4D).
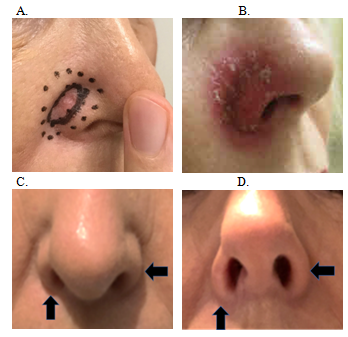
Figure 4 Chronology of Case 1. (A) Planning photo of 15 mm BCC in right nasal groove showing internal full line outlining the gross tumour volume (GTV)21 and the outer dotted line delineating the SXRT field. (B) Moist desquamation at the end of RT. (C) Seven months post RT. Anterior projection – vertical arrow shows thinned right nasal ala compared to the unirradiated left, shown with horizontal arrow. (D) Seven months post RT. Inferior projection – vertical arrow shows thinned right nasal ala compared to the unirradiated left, shown with horizontal arrow.
Case 2
A 76-year old female with no comorbidities presented with a 15 mm lesion of the right nasal ala groove previously treated with cryotherapy (Figure 5A). Histopathology showed an infiltrating nodular BCC 1.5 mm thick. She self-referred for definitive RT. She was treated with SXRT 100 Kv, with HVL 3.95 mm, with a source-to-surface distance of 30 cm dosed to the surface, collimated with a 2 cm cone. Her nose was packed with a water-equivalent bolus prior to therapy with an internal nose shield. She was treated over 27 days to a total dose of 50 Gy in 20 fractions, that is, at fraction sizes of 2.5 Gy. Moist desquamation was noted at the end of treatment (Figure 5B). Eight months post treatment she reported her right nasal ala to be thinned compared to the left (Figure 5C‒D).

Figure 5 Chronology of Case 2. (A) Planning photo of 15 mm BCC in right nasal groove showing internal full line outlining the GTV21 and the outer dotted line delineating the SXRT field. (B) Moist desquamation at the end of RT. (C) Eight months post RT. Anterior projection – vertical arrow shows thinned right nasal ala compared to the unirradiated left, shown with horizontal arrow. (D) Inferior projection – vertical arrow shows thinned right nasal ala compared to the unirradiated left, shown the horizontal arrow.
Case 3
A 78-year old male with no comorbidities presented with a lesion measuring 10 mm in diameter on the right nasal ala groove that had progressed over several months despite cryotherapy and cautery (Figure 6A). Histopathology of a punch biopsy showed an eroded nodular BCC 1.6 mm thick. He was referred for definitive RT. The lesion was treated with SXRT 100 Kv, HVL 3.95 mm, with a source-to-surface distance of 30 cm dosed to the surface collimated with a 2.5 cm cone. His nose was packed with water-equivalent bolus prior to therapy. He was treated over 28 days to a total dose of 50 Gy in 20 fractions, that is, at fraction sizes of 2.5 Gy. He had moist desquamation at the end of his treatment. At five weeks post-RT he was starting to experience thinning of the right nasal ala compared to the unirradiated left side (Figure 6B).
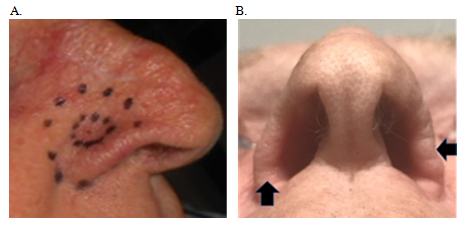
Figure 6 Chronology of Case 3. (A) Planning photo of 1.0 cm BCC in right nasal groove showing internal line outlining the gross tumour volume (GTV) (ICRU 50 ref) and the outer dotted line delineating the SXRT field. (B) Five weeks post RT - Inferior projection – vertical arrow shows already thinning right nasal alar compared to the unirradiated left, shown with horizontal arrow.
We present three cases of self-reported thinning of the nasal ala months following completion of successful mildly hypofractionated definitive SXRT for skin cancer, which had a negative impact on function and cosmesis of the patients involved, and thus on their quality of life. The nose has important functional and cosmetic value. RT is known to have excellent oncological outcomes comparable to surgery when used as primary treatment for SCCs and BCCs.3,10,11 Whilst some patients may not be surgical candidates due to comorbidities and age, for many others RT is being chosen as primary therapy for certain anatomical areas including the eyelids, nose and lips, due to the improved functional and cosmetic outcomes from the tissue conservation that RT provides.
There is a wide variation in both the total dose and dose per fraction involved in radiotherapy for skin cancer, and non-standard fractionation is common. This is due in part to the lack of robust evidence for radiation therapy in skin cancer.12 There is some evidence that fractionation impacts the development of late effects following RT. Reducing the dose per fraction has been studied in skin and other internal cancers. Early evidence suggests that reducing the dose per fraction improves the repair of sublethal damage in late responding tissues, such as the human skin.13,14 Standard fractionation of 1.8-2 Gy per fraction has been devised to allow for this repair and survival of normal late responding tissues, while still being lethal to malignant cells. Recent meta-analyses have demonstrated that both standard fractionation and hypofractionation have similar oncological outcomes when used as primary therapy for non-melanoma skin cancers.9,15,16 However, when compared with standard fractionation, hypofractionation can lead to more skin atrophy and fibrosis. Therefore, especially in younger patients and in areas of the face with high cosmetic value, such as the nasal alar, our case series provides some evidence that standard fractionation should be used, especially as longer term cosmesis may worsen over time.16
Decreasing the total dose delivered to target tissues may also assist in reducing late effects on the skin. Variation in radiosensitivity has been previously observed, for example in extensive skin field cancerisation (ESFC), and indicates that dosing regimens should be personalised to account for this.17 Unfortunately, there is no reliable clinically available personalised radiosensitivity test that can help to identify such individuals. Alternatively, the use of agents or factors to sensitise skin have also been explored and may also be used to reduce the total dose required, such as hyperthermia, which increases radiation sensitivity and also improves tumour oxygenation for subsequent radiation treatments.18 Adaptive split-course RT (ASCRT) is one possible novel method of achieving an overall lower dose and less fractions that has been trialled in the treatment of locally advanced skin cancers in the frail and elderly.19,20 ASCRT is delivered in two phases, both given over five days, separated by an eight week break. This allows for tumour regression and therefore a smaller phase two tumour volume. This regimen reduces visits to a radiotherapy centre, making treatment completion easier for the frail and elderly. ASCRT has been shown to have good oncological outcomes, but it is currently too early to assess long term functional and cosmetic outcomes.
In these three cases, treating non-melanoma skin cancers of the nasal ala with definitive RT using mild hypofractionation regimens, self-reported alar thinning was identified within a year. When function and cosmesis are important end points for patient quality of life, standard fractionation may require consideration.
Permission from the patients involved was obtained prior to formulating of this manuscript. These patients were the motivation behind this paper, as all patients had expressed concern about their cosmetic outcomes. The authors would also like to thank Aileen Eiszele of A&L Medical Communications for manuscript preparation and review.
The authors have no conflicts of interest to declare.

©2020 Butcher, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.