International Journal of
eISSN: 2574-8084


Case Report Volume 10 Issue 1
1University of Lubumbasi Faculty of Medicine, DR. Congo
2Neurodiagnostic Center of Lubumbashi, DR. Congo
3Neuropsychiatric Center Doctor Joseph Ghislain, Brothers of Charity, DR. Congo
Correspondence: Patrice NTENGA, University of Lubumbasi Faculty of Medicine, DR. Congo
Received: January 19, 2023 | Published: February 8, 2023
Citation: NTENGA P. Kiza-ntenga syndrome. Int J Radiol Radiat Ther. 2023;10(1):8-10. DOI: 10.15406/ijrrt.2023.10.00346
The nervous system (NS) in its complexity still has some surprises in store for us. The developmental disorder of the NS during the embryonic period is at the origin of malformations of cortical development (CDM) which represent a major cause of mental and motor handicaps as well as severe epilepsy.
Many anomalies of the latter (SN) are not yet described and will continue to be described over time given the significant advances in science observed in recent years. We describe here a syndrome called Kiza-Ntenga syndrome which would be strongly of embryonic origin and which is made of right cerebral hemiatrophy, facial asymmetry with prominence of the face ipsilaterally to the cerebral hemiatrophy, right hemierythroderma, secondarily generalized focal seizures, slight regression in speech and walking. And so, Kiza designates the name of the patient and Ntenga which designates the name of the author who described him.
Keywords: Kiza, Ntenga, syndrome, right cerebral hemiatrophy, right hemierythroderma, right facial hemiprominence, secondarily generalized focal seizures
We report in this paper the picture of a child K, male, 1-year-old, weighing 9 kg, received at the neuropsychiatric center. Born at term, normal delivery with a birth weight of 4,100 kg. One day later, he was put in an incubator without oxygenation. He is the 7th in a family of 7 children with a history of malformation reported in the family or epileptic seizures. Her mother reports regression in milestone (speech and walking). On examination, we find cranial dysmorphism made up of an asymmetrical head with the right hemiface more prominent than the left (Figure 1), right hemicorporal erythroderma from head to foot taking the inner and posterior face (Figure 1) and describing an atypia by taking half of the sole of the right foot (Figure 2) while the left hemibody is of normal colour. There are left hemicorporal tonic-clonic convulsive seizures which become generalized secondarily and are undergoing. A treatment consisting of valproic acid syrup at 30 mg/kg and injectable dexamethasone at 1 mg/kg made it possible to control the attacks after a failure of treatment with phenobarbital which was administered at the referral hospital. A performed sleep EEG showed spikes and some wave spikes localized to the rigth hemisphere, A performed cerebral computed tomography shows on an axial section a cerebral hemiatrophy of the right hemipshere (Figure 3) with a double cerebral cortex in the frontal region and polymicrogyria (Figure 4). A basic biological assessment carried out and not contributory.
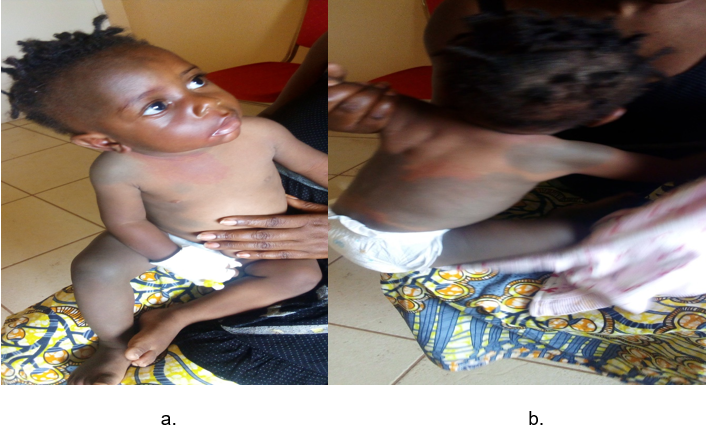
Figure 1 Right hemicorporal erythroderma anterior surface with prominence of the right hemiface (a) and posterior surface (b).
After stabilization (stopping of convulsive seizures under treatment), the examination revealed an attentive child, with good ocular pursuit, without motor deficit, well-toned, able to stand with support and able to walk but dysphasic. The rest of the exam was unremarkable. This table describes, not only that it is out of the ordinary but deserves to be described as a syndrome in its own right from the group of epileptogenic encephalopathies; which we call Kiza – Ntenga syndrom (Kiza to designate the name of the patient who suffered from it, and Ntenga to designate the name of the author who describes it).
This present syndrome is congenital and secondary to a developmental anomaly of the nervous system. This etiological hypothesis is the most plausible and is supported by counter arguments:- the presence in the child of an anomaly of gyration (polymicrogyria) (Figure 4); process which begins from the 20-22 week of pregnancy, as well as the presence of a double cortex in laminar form which is only a consequence of migration disorders which for some authors would be of genetic origin1 and/or related to the environment.2,3 It is known that during inside-out migration and the establishment of cortical lamination, neurons can differentiate in abnormal or heterotopic position and can be grouped into subcortical nodules or form a band called double cortex syndrome.4-8
In addition, laminar subcortical heterotopias (HLSC) are considered to be a moderate form of smoothencephaly because they share their genetic bases and their physiopathological mechanisms.9
The unilateral pigmentation anomaly (Fig a, b) given the common embryological origin of the nervous system and the skin. The differential diagnosis is then made here with Sturge Weber's disease (SWD) which is encephalotrijeminal angiomatosis, a neuro-oculo-cutaneous syndrome associating a facial plane angioma, a leptomeningeal angioma and ocular abnormalities. This condition (Sturge Weber's disease) is usually non-familial, sporadic affecting all races and also both sexes with a male predominance according to some authors. Its frequency is estimated at one case per 10,000 births.10-13
In fact, this syndrome has several clinical varieties. A classification is proposed by Roch and Coll which describes 3 forms of SWD: type 1 (classic SWD) with intracranial and facial manifestations, type 2 with only facial involvement without central changes, type 3 similar to type 1 but without facial lesions. Type 2 seems more of a dermatological curiosity than a variant of SWD, even though the lesions may have similar etiologies.14,15
From this classification, it emerges that Kiza – Ntenga Syndrome does not appear in a class, which makes this table a new entity which must have its place in current literature.
The hemiatrophy of the right cerebral hemisphere in our patient proves a complete migration of neurons to the definitive site, but the lack of development or maturation would explain this cerebral anomaly. And therefore, this would be neither of inflammatory origin as in Rasmussen's encephalitis which constitutes a differential diagnosis, nor of degenerative origin.
Rasmussen's encephalitis is a sporadic and rare pathology, the incidence of which is estimated at 2.4 cases per 107 inhabitants per year.16 Without literally demonstrated predominance of sex or ethnicity. It is specifically a chronic disease that affects young children, whose median age of onset is 6 years.17,18
Clinically, Rasmussen's encephalitis is characterized by a picture of drug-resistant unilateral focal epilepsy with progressive deterioration of the functions supported by the affected hemisphere (motor, sensory, cognitive or visual functions).
In our patient a regression of language and walking was noted. Moreover, Rassmussen is of inflammatory origin while Kiza-Ntenga syndrome is an embryopathy. This makes all the difference between the two entities although we find a cerebral hemiatrophy as an essential element of intersection between the 2.
The nature of hemispheric functional specialization has been the subject of much research carried out both in patients suffering from unilateral brain lesions, in patients who have undergone commissurotomy in order to control drug-resistant epilepsy, and in neurologically healthy subjects.19
Knowing the concept of hemispheric specialization in a human being, the right hemisphere with its properties of intervening in the visuospatial function, in the production of negative emotions, in the processing of abstract sentences or even understanding double-meaning sentences and metaphors. It is therefore important to follow this kind of syndrome to counterbalance with the notion of cerebral plasticity in order to see if there would be a cerebral organization in the sense of a transfer of information processing skills from the hemisphere. “sick” (right) to healthy hemisphere (left).
The literature shows that it is possible that at the age of three years, the division of attention has not yet been established between the two hemispheres and that a reorganization has been established spontaneously and therefore what would prevent the appearance of certain neuropsychological disorders.20
Any child having convulsive seizures and who has experienced a stoppage and/or a regression in the acquisition of his higher functions; whose observation shows a right facial hemiprominence with an ipsilateral anomaly of the skin pigmentation (erythroderma), a cerebral imaging must be requested in search of a right cerebral hemiatrophy to make the diagnosis of Kiza-Ntenga Syndrome. Dual therapy is the first option for controlling seizures.
None.
All authors declare that there is no conflicts of interest.

©2023 NTENGA. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.