International Journal of
eISSN: 2574-8084


Technical Paper Volume 11 Issue 6
Genesis Cancer Care, Australia
Correspondence: Huong Ho, Department of Radiation Oncology, Genesis Cancer Care Ringwood, Australia, Tel +61 38870 3300
Received: December 16, 2024 | Published: December 26, 2024
Citation: Ho H, Thomson A, Bolton D, et al. Iodinated hydrogel as a tissue fiducial marker for image-guided radiation therapy in bladder cancer. Int J Radiol Radiat Ther. 2024;11(6):171-175. DOI: 10.15406/ijrrt.2024.11.00407
Aim: This is a prospective study reporting on the safety and feasibility of an iodinated hydrogel tissue fiducial marker (IH TFM) for image guided radiation therapy in the treatment of muscle invasive bladder cancer.
Materials and Methods: From September 2015 to July 2017, four patients diagnosed with muscle invasive unifocal transitional cell carcinoma (TCC) bladder cancer were included in the study. Under general anaesthetic, patients underwent cystoscopic injection of IH into their tumour base. Patients subsequently underwent image guided RT. The total prescription was 64.0 - 66.0 Gy in 2.0Gy per fraction. Daily online cone-beam CT (CBCT) matching to IH TFM were performed throughout the course of radiation therapy (RT) to verify the extent of daily treatment shifts. IH volume, its stability and visibility were also evaluated.
Results: The volume of IH TFM remained consistent over the course of bladder radiotherapy. IH TFM match recorded the largest variations in the supero-inferior (SI) and antero-posterior (AP) directions with the largest geometrical shift of 5 mm was recorded. If bony landmark was used, a margin of up to 17.4 mm in the AP direction would be required to ensure adequate clinical target volume (CTV) coverage. In this study, we found IH TFM to be well tolerated and feasible, with no major adverse events noted as a result of injection.
Conclusion: This study demonstrates that IH TFM can be safely injected into the bladder mucosa and can be considered as a fiducial marker for bladder cancer.
Keywords: Bladder cancer, radiation therapy, iodinated hydrogel, tissue fiducial marker, bladder sparing
Bladder cancer is the fifth leading malignancy globally and its incidence is increasing.1 In 2019, it was the twelfth most commonly diagnosed malignancy, accounting for an estimated 2.0% of all cancers diagnosed in Australia.2 Although definitive chemo-radiation therapy (CRT) is an excellent organ preserving treatment option, radical cystectomy with neo-adjuvant chemotherapy remains the standard treatment option for patient diagnosed with muscle invasive bladder cancer.3-5 CRT post transurethral resection of bladder tumour (TURBT) has been shown to achieve a five-year overall survival of 55 % - 67 %.6-9 The standard RT technique consists of treating the whole bladder but this may lead to higher toxicity rates due to the significantly larger bladder volumes irradiated to the full prescription dose.10 This can be problematic in the older cohort of patients usually referred for CRT as they have multiple existing co-morbidities that will make it more challenging for both patients and health clinicians to manage. Partial bladder RT has shown promise with no impact on the local control when compared to standard whole bladder RT.11,12 The use of partial bladder RT reduced overall toxicity and improved QoL. However, accurate and reliable delineation of the gross tumour volume (GTV) is challenging due to the uncertainty in the daily variation of bladder volumes.
The use of fiducial markers in bladder cancer has previously been explored. Lipiodol has been used a tissue fiducial marker (TFM),13-16 however it is known to diffuse widely into the surrounding bladder mucosa. This reduces its value as a TFM as it can be difficult to clearly identify the GTV on CT simulation. Iodinated Hydrogel [IH (TraceIT)] has recently attracted much interest as an alternative TFM. IH is an absorbable radiopaque hydrogel consisting of iodinated polyethylene glycol (PEG) hydrogel particles. The PEG iodination property enables IH to be visible on both CT and CBCT without any artefacts. Once injected, IH will remain in-situ for up to three months prior to undergoing hydrolysis and clears from the body after seven months. In this study, we report on the feasibility and the safety of IH TFM for image-guided radiation therapy (IGRT) in bladder cancer and its potential role in the management of bladder cancer.
Our institutional research review board approval was obtained prior to commencement of this study. All participants provided written medical informed consent prior to undergoing any therapeutic procedures.
From September 2015 to July 2017, four patients with unifocal pT1-T2N0M0 muscle invasive bladder cancer were included in this study. Median age was 88 years (ranging 80-90 years). All patients had a TURBT prior to commencement of CRT. Patients were excluded from the study for multifocal bladder tumour, distant metastatic disease, total hip replacement, allergy to Iodine or known active metabolic disorder.
IH InjectionUnder general anaesthetic, a rigid 20 F injection cystoscope was introduced into the bladder. Using a 23G flexible Cook Williams cystoscopic injection needle, 0.3 - 0.4ml of IH was injected into five locations at least 1cm circumferentially around the tumour bed (Figure 1). A total of 2 ml of IH tissue marker was injected into the bladder mucosa.
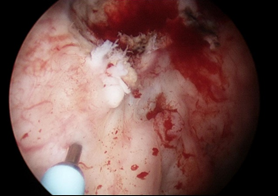
Figure 1 Bladder tumour clearly visible (red circle) with IH TFM injection 1cm from tumour using cystoscopic injection needle (blue arrow).
Pelvic planning computed tomography (CT) scan for volumetric modulated arc therapy (VMAT) was performed approximately 3 - 5 days post IH injections. All patients were required to have two separate CT simulation scans, the first with a comfortably full bladder and the second with an empty bladder. For the full bladder CT scan, patients were instructed not to empty their bladder 45 minutes prior to their appointment time. They were also told to drink 600 mL of water. Patients were then instructed to empty their bladder prior to the second (empty bladder) CT scan. All patients were scanned in the supine position with strict instructions for both bladder and bowel preparations to follow prior to both CT simulation and for their daily treatments. CT simulation was performed using a Siemens Somaton CT Scanner (Siemens Healthineers, Erlangen, Germany) with 3.0 mm slices. All CT images were then transferred to MIM v 6.8.9 (MIM Software Inc. Cleveland, OH) for contouring prior to transferring to Pinnacle v 9.8 (Phillips Radiation Oncology Systems, Fitchburg, WI) treatment planning system (TPS) for dosimetry.
The GTV was defined as the primary tumour or TURBT cavity identified on cystoscopy and demarcated by the IH TFM injection. The partial bladder clinical target volume (CTV) for partial bladder RT was created by adding a 1cm bladder wall radial margin around the GTV. The partial bladder planning target volume (PTV) was created by adding a uniform margin of 1.0 cm around the partial bladder CTV.
The whole bladder CTV consisted of the entire empty bladder structure. The whole bladder PTV was created by adding a margin of 1.0 cm to the whole bladder CTV.
All patients received the initial phase 1 partial bladder RT with a radiation dose of 14–16 Gy in 7 - 8 fractions delivered to the partial bladder PTV, followed by phase 2 whole bladder RT of 50 Gy in 25 fractions delivered to the whole bladder PTV utilising 6 MV VMAT technique.
Matching guidelinesTreatment was delivered daily, five days per week on a Varian True Beam linear accelerator equipped with kilovoltage (kV) CBCT capabilities. Our departmental imaging protocol was daily on-line CBCT matched to IH TFM contours using the soft tissue alignment algorithms. Daily on-line CBCT match to the IH TFM contours and off - line matching to bony landmarks were performed with off-sets relative to skin tattoos were recorded in three directions: LR = Left (+) / Right (-); SI = Superior (+) / Inferior (-) and AP = Anterior (+) / Posterior (-). All shifts were recorded in mm. No couch rotation shifts were performed. Mean and standard deviation (SD) of the shift differences were calculated for all patients over all measured fractions to derive the overall mean systematic error (OM), standard deviation (SD) of systematic error (Σ) and SD of random error (σ), as previously described by van Herk.17
IH and bladder assessmentPhase 1 partial bladder CBCT images were imported back to Pinnacle TPS for assessment of bladder volume. Each IH TFM was contoured and measured for stability and their position relative to the bladder fillings. Additionally, IH TFM volume and the visibility were also assessed to evaluate any changes in the IH TFM as a function of time over the first 7 -8 fractions and their relationship with bladder volume for the full bladder treatment.
IH TFM was found to be straightforward to inject cystoscopically into the bladder mucosa. None of our patients reported any post-operative complications such as bleeding, infection or allergic reactions following the IH injections.
VisibilityAll IH TFM were clearly visible at CT planning and throughout the first 7 - 8 fractions of the partial bladder RT (Fig 2). In addition, there was no significant difference in correlations between the IH TFM visibility and bladder volume. See Table 1.
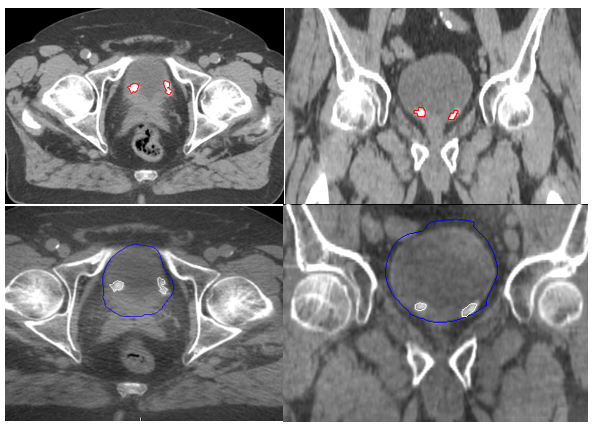
Figure 2 TraceIT® Blebs at planning CT in axial and coronal views (A and B) and during partial treatment in axial and coronal views (C and D).
|
Patient No |
No. of injected TraceIT® |
Mean TraceIT® vol on CT (Range) (cc) |
Bladder vol on CT (cc) |
Mean TraceIT® vol on CBCT (Range) (cc) |
Mean bladder vol on CBCT (cc) (range) |
|
1 |
5 |
0.4 (0.2-0.6) |
202 |
0.4 (0.2-0.5) |
183 (107 – 279) |
|
2 |
5 |
0.3 (0.2-0.5) |
287 |
0.3 (0.2 – 0.4) |
244 (120-360) |
|
3 |
5 |
0.4 (0.2 – 0.5) |
333 |
0.4 (0.3-0.5) |
216 (145 – 334) |
|
4 |
5 |
0.3 (0.3 – 0.4) |
238 |
0.3 (0.2-0.3) |
233 (182 – 278) |
Table 1 Bladder and TraceIT® blebs visibility comparison between CT and partial RT
A total of 30 CBCT images of partial bladder RT were reviewed and assessed. Table 2 shows the magnitude of errors recorded in all three directions for matching both on-line to IH TFM and off - line to bony landmarks.
|
|
LR |
SI |
AP |
|||
|
|
IH TFM |
Bony |
IH TFM |
Bony |
IH TFM |
Bony |
|
Mean (mm) |
-0.2 |
0.0 |
-1.5 |
-0.8 |
1.4 |
1.6 |
|
Systematic error (mm) |
2.8 |
1.8 |
4.1 |
3.6 |
5.1 |
1.0 |
|
Random error (mm) |
3.7 |
3.3 |
4.7 |
3.6 |
5.4 |
3.0 |
Table 2 Magnitude of errors between IH TFM and bony matching
Based on the results presented in Table 3, both the AP and SI recorded the greatest variations and if patients were to match to bony landmarks during partial bladder RT, a margin of 10.0 mm and 17.4 mm in the SI and AP directions respectively, would be required to ensure sufficient coverage of the CTV.
|
|
LR |
SI |
AP |
|
Overall mean¹ (mm) |
-0.2 |
-0.7 |
-0.1 |
|
SD mean² Σ (mm |
1.1 |
2.9 |
5.5 |
|
RMS SDs³ σ (mm) |
2.9 |
3.8 |
5.2 |
|
Margin = 2.5Σ + 0.7σ (mm) |
4.7 |
10.0 |
17.4 |
Table 3 Magnitude of random and systematic errors
Overall mean, group systematic errors; ² SD mean, standard deviation of systematic error; ³ RMS SDs, standard deviation of random error
Both gold seed fiducial markers and Lipiodol have been used as fiducial markers in the bladder RT settings. However, gold seed migration and intravesical diffusion of Lipiodol has limited its use.15,16,18
In this present study, we have found IH to be a safe and effective TFM for IGRT. IH was found to be straightforward to inject cystoscopically into the bladder submucosa. None of our patients reported any post-operative complications. IH has a comparable tissue density value of 1.02 g/cm3 making it radiopaque on CT and CBCT with each small volume IH TFM clearly visible without any imaging artefacts (Figure 2). However, we were not able to identify the IH TFM on kV imaging. Each IH TFM was successfully injected into the bladder submucosa with no intravesical diffusion, migration or loss seen. The size and shape of the IH TFM remained clearly visible and stable for the entire duration of partial bladder RT with minimal loss of volume (Table 1).
The major problem with partial bladder RT is the inability to clearly and reliably delineate the tumour or TURBT cavity on CT planning. Even if the mass can be identified on a CT scan, any attempt at partial bladder RT will require larger GTV margins to account for the inherent inaccuracy of delineating the primary tumour. This can be resolved with the use of IH TFM, where the tumour or TURBT cavity is identified under direct vision cystoscopically and marked with IH at a distance of 1cm radially around its periphery.
In addition, once the CTV is drawn, without the use of an effective TFM, we will also require a larger PTV margin to account for the inter-fractional variation seen as a result of different bladder volumes encountered during treatment. This variation occurs due to different level of bladder fillings on a day to day basis, which may not always replicate the bladder volume at CT simulation despite the strict bladder instructions. Pos et al.19 reported geographical misses in up to 65 % of their patients, with the GTV outside the PTV margins at least once during RT. The use of IH TFM can minimise the risk of a geographical miss. When compared with anatomical bony matching, IH soft tissue matching has identified a larger variation in set up errors in all directions, especially in the antero-posterior and supero-inferior directions. Sondergaard et al.20 has also identified the largest set up errors in the AP and SI directions with an average of 5 mm geometrical shift using Lipiodol markers. This is comparable to our results and if we were matching to bony landmarks, a geometrical shift of 5 mm in the AP direction would be required to adequately cover the primary tumour (Table 2).
Bladder organ motion and its impact on treatment margins have posed a challenge over the years due to uncertainty in daily variation of bladder volume, leading to potential geographical miss of the target volume, thus compromising local tumour control. Larger treatment set up margins are required but at the detriment of increasing the risk of radiation damage to the surrounding normal tissue. Several studies have investigated and proposed a margin of 20–25 mm if standard bone matching is used to account for set up margins without compromising the target coverage.19,21-23 Based on our calculations, if patients were aligned according to bony landmarks, a margin of 4.7 mm in the LR; 10 mm in the SI and 17.4 mm in the AP directions would be required to ensure the minimum dose to the CTV is at least 95 % of the target dose in 90 % of patients. However, increasing the posterior margin to 17.4 mm would certainly increase the dose to the rectum and consequently increase rectal toxicity. Using IH TFM for IGRT can eliminate most of the errors associated with standard bone matching, thereby ensuring better dose coverage of the CTV without increasing the surrounding normal toxicities.
Given this was a small sample size, further investigation of this technique should be done in a larger sample to ensure that inter-patient variability is appropriately demonstrated, allowing for more accurate assessment of this technique.
We have demonstrated that IH TFM can be safely injected in the bladder mucosa. IH TFM can facilitate partial bladder RT as it allows the accurate delineation of the tumour or TURBT cavity and improves IGRT by minimising the current recommended PTV margins with standard bone matching.
None.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.

©2024 Ho, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.