International Journal of
eISSN: 2574-8084


Research Article Volume 6 Issue 4
Faculty of Medicine, Department of Radiology, Airlangga University, Indonesia
Correspondence: Lies Mardiyana, Faculty of Medicine, Department of Radiology, Universitas Airlangga, Indonesia, Tel +62 877-0333-0249
Received: May 31, 2019 | Published: August 27, 2019
Citation: Seto AAB, Mardiyana L. Intrinsic subtypes of breast cancer in malignant speculating mass on mammography. Int J Radiol Radiat Ther. 2019;6(4):144-148. DOI: 10.15406/ijrrt.2019.06.00236
Background: Speculated mass is one of the indications for malignancy in mammography. Breast cancer can be divides into four intrinsic subtypes, Luminal A, Luminal B, HER2+ and basal-like/triple negative. Each with differing prognosis, Luminal A and B has a better prognosis than HER2+ and the worst prognosis being the basal-like subtype.
Objective: To describe each intrinsic breast cancer subtypes that have the appearance of malignant speculated mass in mammography.
Material and method: This was a retrospective cross sectional study that used medical record data using consecutive sampling between January 2017 and December 2018 in Dr. Soetomo Surabaya General Hospital. The mammography images of speculated mass were reviewed by a breast imaging consultant of radiology. The pathological results were obtained from the medical record data.
Result: We found that breast cancer with a mammographic appearance of speculated mass, the majority of them were of Luminal A subtype, followed with Luminal B, HER2+ and Basal-like subtypes
Conclusion: In this study, we found the majority of breast cancer with a mammographic appearance of speculated mass was of the subtype Luminal A.
Keywords: malignant mass, mammography, intrinsic subtypes, luminal A, luminal B, HER2+, Basal-like/triple negative
Breast cancer is the most commonly diagnosed cancer in women. In 2016, around 14.94 million new cases of breast cancer were diagnosed and resulted in around 8.2 million deaths worldwide. Estimates of new breast cancer cases are 1.78 million, accounting for 26% of all new female cancer cases (6.89 million cases), and the estimated mortality is 460,000: 13.3% of all female cancer deaths. The incidence of breast cancer is 25.8%, and the mortality rate is 13.3% worldwide.1 Breast cancer often shows intratumoural heterogeneity, so the pattern of breast cancer on mammography has a variety of variations.2 Mammography is one of the imaging modalities that uses x-ray to evaluate breast tissue, and has become one of the most significant diagnostic approaches in evaluating breast mass, with a sensitivity level of between 60-90% and a specificity of about 93%, final diagnosis based on examination histopathology.3,4 On mammography, mass is defined as a lesion which appears in two projections with its morphological characteristics of shape, edge and density.5 Some typical mammographic images can reflect the nature and biological behaviour of tumours that can provide valuable information to the clinician.3 One important characteristics on mammography is speculation in mass which is the appearance of mass with a central part that is dense/opaque, the edges of which appear several linear lines called spicules.5 Mammographic images that predict malignancy include masses with spicule margins (PPV 81%) and irregular forms (PPV 73%).4 Based on ACR-BIRADS 2013, the mass of spicules on mammography is one of the findings of classic breast cancer with a high suspicion of malignancy (possibility of malignancy> 95%).6
Speculated mass on mammography is a typical feature of invasive breast cancer and is an important criterion in the diagnosis of disease. In addition, several studies have shown that speculated mass on mammography can also predict prognostic factors and predictive factors that play a major role in therapeutic management.3,7 Prognostic factors are factors that may predict the risk of recurrence or death from breast cancer. While predictive factors are the factors that can be predict possibility of therapeutic response. Prognosis factors and predictive factors are the basis of breast cancer management strategies so that the risk of recurrence and death from breast cancer can be prevented.8 Status of estrogen receptor (ER), progesterone receptor (PR), human epidermal growth factor receptor 2 (HER2) are amongst the prognostic and predictive factors.9 Based on the three immunohistochemical markers mentioned above along with the KI67 protein (protein marker for proliferation), breast cancer is divided into four intrinsic subtypes, Luminal A, Luminal B, Triple-negative/basal likes and HER2/NEU. The research conducted by Liu et al.7 in 2016 showed that Luminal A subtype (ER/PR positive, negative HER2, Ki67<14%) is a very important factor for the formation of speculated masses on mammography compared with Luminal B subtypes (ER/PR positive, HER2 negative or positive, Ki67≥14%) or on a basal-like tumour/triple negative (ER/PR negative, HER2 negative) that does not show a speculated border even though the exact cause is still unknown.7
The findings of the study conducted by Moriuchi et al.10 in 2015 shows that the invasion of cancer cells in marginal adipose tissue is an important factor for the visualization of speculated masses on mammography.10 Another study conducted by Evans et al.11 in 2006 with the result that the speculated mass was significantly associated with the survival rate of breast cancer patients due to the strong correlation between low histological degree lesions and spicules on mammography.11 Taneja et al. in 2008 showed that the majority of the speculated mass was a Luminal A subtype that was correlated with a good prognostic value.12 In this study, we aim to describe the intrinsic subtypes of breast cancer that has an appearance of spiculating mass.
This was a retrospective cross sectional study that used medical record data using consecutive sampling between January 2017 and December 2018 in Dr. Soetomo Surabaya General Hospital. The inclusion criterions are:
With exclusion criterions are:
The aim of this research is to describe the intrinsic subtypes of breast cancer which has an appearance of spiculated mass on mammography. There were 59 patients that met the inclusion criterions at DR. Soetomo General Hospital, Surabaya between January 2017 and December 2018 (Figure 1−4). The youngest subject is 34 years old and the oldest being 73 years old with the average age of 52.1 years old. We divided the patients into three age groups, below 40 years old, 40-50 years old and above 50 years old. The average age of subtype Luminal A were the oldest with 53.6 years old, 52.9 years old in Luminal B, 47.4 years old in the basal-like and the youngest of which 46.8 years old in the HER2+subtype (Table 1) (Figure 5).
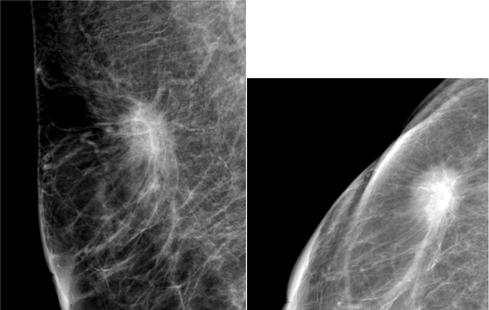
Figure 1 The mammographic appearance of spiculated mass in subject with pathological subtype Luminal A.

Figure 2 The mammographic appearance of spiculated mass in subject with pathological subtype Luminal B.

Figure 3 The mammographic appearance of spiculated mass in subject with pathological subtype Luminal C.

Figure 4 The mammographic appearance of speculated mass in subject with pathological subtype Luminal D.
|
Age group |
Frequency (n) |
Percentage (%) |
|
<40 years old |
5 |
8.5 |
|
40-50 years old |
22 |
37.3 |
|
>50 years old |
32 |
54.2 |
|
Total |
59 |
100 |
Table 1 Distribution of age groups in breast cancer with mammographic appearance of speculating mass
From the 59 samples, 48 patients has a pathological result of invasive ductal carcinoma, 8 patient with invasive lobular carcinoma and only 1 patient has a pathological result of ductal carsinoma in situ. There were two patient with other invasive pathological type (tubular carcinoma and metaplastic carcinoma) (Table 2) (Figure 6). Based on pathological grading, most (29 subjects) has grade III cancer, 22 subjects has grade II and only 8 subjects has first grade cancer (Figure 2). Most (27 subjects) has Luminal A subtype, 21 subjects has Luminal B, 6 subjects has HER2+ and only 5 subjects has the basal-like subtype (Table 3) (Figure 7). The results showed that within the Luminal A subtype group, most has invasive histopathology of ductal carcinoma 77.8% (21 people), grade II was 44.4 %% (12 people), and in the age group over 50 years was 66, 7% (18 people). Within the Luminal B group, most has invasive ductal carcinoma histopathology 81% (17 people), grade III is 42.9% (9 people), and in the age group of more than 50 years is 52.4% (11 people). Within the HER2+ subtype group, all has invasive ductal carcinoma, grade III amounted to 66.7% (4 people), and in the 40-50 years age group as many as 50% (3 people). Within the basal -like subtype group, most has invasive ductal carcinoma 60% (3 people), grade 3 100% (5 people), and in the 40-50 years age group as many as 60% (3 people) (Table 4).
|
Pathological type |
Frequency (n) |
Percentage (%) |
|
Invasive lobular carcinoma |
8 |
13.6 |
|
Invasive ductal carcinoma |
48 |
81.4 |
|
Tubular carcinoma |
1 |
1.7 |
|
Metaplastic carcinoma |
1 |
1.7 |
|
Ductal carcinoma in situ |
1 |
1.7 |
|
Total |
59 |
100 |
Table 2 Frequency of each pathological type in breast cancer with mammographic appearance of speculating mass
|
Subtype |
Frequency (n) |
Percentage (%) |
|
Luminal A |
27 |
45.8 |
|
Luminal B |
21 |
35.6 |
|
HER-2+ |
6 |
10.2 |
|
Basal-like |
5 |
8.5 |
|
Total |
59 |
100 |
Table 3 Distribution for each breast cancer subtypes with mammographic appearance of speculating mass
|
Luminal A |
Luminal B |
HER2+ |
Basal-like |
|
|
Pathological type |
||||
|
Invasive lobular carcinoma |
3(11.1%) |
4(19%) |
0(0%) |
1(20%) |
|
Invasive ductal carcinoma |
21(77.85) |
17(81%) |
6(100%) |
3(60%) |
|
Tubular carcinoma |
1(3.7%) |
0(0%) |
0(0%) |
0(0%) |
|
Metaplastic carcinoma |
0(0%) |
0(0%) |
0(0%) |
1(20%) |
|
Ductal carcinoma in situ |
1(3.7%) |
0(0%) |
0(0%) |
0(0%) |
|
Total |
27 (100%) |
21(100%) |
6(100%) |
5(100%) |
|
Grade |
||||
|
Grade I |
4(14.8%) |
4(19%) |
0(0%) |
0(0%) |
|
Grade II |
12(44.4%) |
8(38.1%) |
2(33.3%) |
0(0%) |
|
Grade III |
11(40.7%) |
9(42.9%) |
4(66.7%) |
5(100%) |
|
Total |
27 (100%) |
21(100%) |
6(100%) |
5(100%) |
|
Age group |
||||
|
<40 years old |
2(9.1%) |
1(3.8%) |
1(16.7%) |
1(20%) |
|
40-50 years old |
3(13.6%) |
13(50%) |
3(50%) |
3(60%) |
|
>50 years old |
17(77.3%) |
12(46.2%) |
2(33.3%) |
1(20%) |
Table 4 Characteristics of each breast cancer subtypes with mammographic appearance of speculating mass
The mammographic findings of mass with speculating edges have been associated with malignancy in previous studies. Some studies have even shown a relationship between the mammographic findings of the mass and histopathological findings. Several studies have shown an association between a spiked mass on mammography and a good outcome (prognosis) in patients.13 Mammographic findings may provide clues about the pathological types of breast tumors including the characteristics of tumor cells and even proliferation of breast cancer cells. This study describes various findings of mammography of patients with a pelvic mass on four histopathological subtypes of breast cancer.4,7 In terms of age, the majority of the subjects of this study (54.2%) were in the group> 50 years. However, the distribution of the younger age average was found in patients with HER2+ and Basal-like subtypes compared to those in the luminal A and B subtype group, the majority of which were in the age group> 50 years. The increase in the frequency of triple negative and HER2+ breast cancer subtypes was found in patients <50 years of age, this is in line with several previous reports. These findings indicate the molecular subtype is one of the strongest prognostic factors.14
In this study, the majority of breast cancer patients with speculated mass in mammography were luminal A subtypes (45.8%), according to previous studies mammographic findings correlated with intrinsic subtypes showed that the form of mass with speculated margin was significantly more frequently detected in Luminal A breast cancer than other subtypes.13 Since the advent of molecular markers examinations, many studies have investigated the features of mammography associated with the presence or absence of hormone receptors. Positive tumor estrogen receptors have been shown to have a higher incidence of speculating mass on mammography and as found in previous studies.12 Most patients with breast cancer that has the appearance of speculated mass on mammography has histopathological results of invasive ductal carcinoma (79.7%), in-line with several previous studies.15 Other studies discuss that the Luminal A subtype breast cancer seems to show more interaction with stromal tissue. Spiculae formations seem to be the result of two steps:
Therefore, the description of the mass spatial on mammography has at least two meanings. First, this is a predictor for the luminal subtype A. Secondly; it is a final stage appearance as a result of adipose tissue invasion by carcinoma tissue.7
In this study, patients with speculating mass on their mammography which has Luminal A subtypes, most were of grade II in accordance with the results of previous studies where breast cancer with Luminal A subtypes had lower histopathological grading than other subtypes. Within the grade I, none were of the basal-like and HR2+. The Triple Negative/Basal-like subtype was found to have histopathological grading II and III. Based on previous research, the features of mammography in the Luminal A and B groups showed more speculated lesions. Tumors in this group (Luminal A and B) represent a more differentiated luminal phenotype (luminal cytokatin CK18 expression).12 Other studies have shown that this differentiation is related to adhesion factors. The loss of adhesion factors in carcinoma cells is thought to play a role in the characteristic histological appearance of invasive carcinoma as a linear cell column that is spread sparsely on the edge of the mass. This more diffuse infiltrative pattern can explain some typical tumor imaging features, such as speculation and distortion. In some studies, adhesion factors correlate with high histological levels. Therefore, adhesion factors can be considered to correlate with the results of this study in spotted breast cancer which has lower grade clinical and histological results.13 This study has several limitations, the relatively small number of subjects in only center may skew the results.
The majority of breast cancer patients with speculated mass in mammography were of Luminal A subtype which was comparable with previous studies yet we found most of the subjects were in grade III which is not in line with most previous studies. Which may elucidate that there are still many biological mechanisms that underlie the relationship between infiltration processes and histopathological characteristics which remain unknown, further research is needed to further look for the relationship of mammographic images to the biological characteristics and histopathology of tumors.
None.
None.
The author declares that there are no conflicts of interest.

©2019 Seto, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.