International Journal of
eISSN: 2574-8084


Research Article Volume 11 Issue 3
National Coalition of Independent Scholars, Battleboro, VT, USA
Correspondence: Marco Ruggiero, MD, PhD, National coalition of independent scholars, 125 putney rd battleboro, VT 05301, USA
Received: June 14, 2024 | Published: June 28, 2024
Citation: Ruggiero M. GcMAF in radiation therapy: identification and molecular characterization of the human GcMAF receptor. Int J Radiol Radiat Ther. 2024;11(3):77-80. DOI: 10.15406/ijrrt.2024.11.00390
Gc protein-derived Macrophage Activating Factor (GcMAF), a powerful immunostimulant endowed with anti-cancer and anti-angiogenetic activities, offers significant advantages when combined with radiation therapy. A stronger immune response improves the effectiveness of radiation therapy by allowing the body to better eliminate residual cancer cells after treatment. In addition, by boosting the immune system, GcMAF mitigates some of the immunosuppressive side effects of radiation therapy, leading to faster recovery. In order to fully exploit the potential of GcMAF in cancer therapy, knowledge of the molecular interactions with its receptor is essential. This study proposes the first extracellular domain (residues 1-34) of the CCR1 protein as the GcMAF receptor. The CCR1 gene, expressed in monocytes and 168 other cell types or tissues, encodes this transmembrane protein. Electrostatic and hydrophobic interactions, along with hydrogen bonds mediate the molecular interactions between the TPT420-GalNAcELAK (or TPK420ELAK) sequences of GcMAF (or Gc2 protein variant) and the TTEDYDTTT sequence of its receptor.
Keywords: radiation therapy; GcMAF; receptor; immune system; cancer; autism; COVID-19
A serum protein of human plasma called group-specific component (Gc protein), or Vitamin D-binding protein, exhibits multifunctionality. It exists in three common variants: Gc1F, Gc1S, and Gc2. Notably, Gc1 carries a specific sugar chain (O-linked trisaccharide) attached to its Threonine residue at position 420 (Thr420). This trisaccharide consists of N-acetylgalactosamine (GalNAc), galactose and sialic acid in this specific order. Selective removal of sialic acid and galactose from Gc1 can convert it into a potent molecule known as Gc protein-derived macrophage activating factor (GcMAF), leaving only GalNAc linked to Thr420. The Gc2 variant lacks this sugar chain entirely since it has Lysine at position 420 instead of Threonine (T420K mutation); nevertheless, the Gc2 variant is able to activate macrophages in a manner superimposable to that of GcMAF.1
GcMAF holds promise as a potential therapeutic candidate for a number of conditions ranging from immunotherapy and antiangiogenic cancer treatment,2-4 to autism,5 neurological side effects of cancer chemotherapy,6,7 and COVID-19.8 More specifically, GcMAF offers significant advantages when combined with radiation therapy. A stronger immune response improves the effectiveness of radiation therapy by allowing the immune system to better eliminate residual cancer cells after treatment. Elimination of residual cancer cells results from direct inhibition of cancer cell proliferation and cancer cell-stimulated angiogenesis as demonstrated by Pacini et al.9 as well as by macrophage-induced cancer cell apoptosis.10 In addition, by boosting the immune system, GcMAF mitigates some of the immunosuppressive side effects of radiation therapy, leading to faster recovery. Finally, in analogy with what observed with anti-cancer chemotherapy,6,7 GcMAF has the potential to minimize the neurological side effects of radiation therapy.11 Therefore, identification of its human receptor and characterization of the molecular interactions between GcMAF and the receptor becomes crucial for understanding its therapeutic potential and developing targeted treatment strategies.
Human Gc protein is known to interact with 65 unique human interactors, 8 of which have the characteristics of, or are associated with receptors. These are: nuclear receptor subfamily 2, group F, member 2; chemokine (C-C motif) receptor 1; cubilin (intrinsic factor-cobalamin receptor); G protein-coupled receptor 18; growth factor receptor-bound protein 2; ligand dependent nuclear receptor interacting factor 1; low density lipoprotein receptor-related protein 2; purinergic receptor P2X, ligand-gated ion channel, 6.13 Of these, only chemokine (C-C motif) receptor 1 (CCR1, also known as HM145, LD78 receptor, Macrophage inflammatory protein 1-alpha receptor (MIP-1alpha-R), RANTES-R) has structure, cellular localization, and function consistent with the known biological and clinical effects of GcMAF. Consistent with the multiplicity of effects exerted by GcMAF in different tissues, CCR1 is expressed in 168 cell types and tissues in addition to monocytes (see Gene expression database Bgee, CCR1 - ENSG00000163823),14 and is the target of at least 11 different ligands.15
CCR1 is a typical G-protein coupled transmembrane receptor constituted by 4 extracellular domains, 7 transmembrane helical domains, and 4 cytoplasmic domains.16 Since GcMAF and Gc2 are large molecules characterized by a great number of hydrophilic, polar amino acids (Figure 1), thus unable to cross the plasma membrane, the only permissible interactions are those with the extracellular domains of the receptor.

Figure 1 Results of alignment of mouse Gc protein (VTDB_MOUSE. Upper line) and human Gc protein (VTDB_HUMAN. Lower line). Polar amino acids are highlighted.
Identification of the extracellular domain and amino acid sequence responsible for binding the active site of GcMAF was made possible by the observation that both ThrGalNAC and Lysine in position 420 of, respectively, Gc1 and Gc2, bind to the active site of the receptor (for details, please see supplementary Figure 3 of Nabeshima et al.1 Therefore, the most likely characteristic of the receptor binding site has to be the presence of negatively charged amino acids able to establish electrostatic and other non-covalent interactions with Lysine and GalNAc.
Figure 2 shows alignment of the sequences of the four extracellular domains of CCR1 where negatively charged amino acids are highlighted. The first domain (1-34; third line, outlined in red) has the highest number of negatively charged amino acids along with an unique sequence TTEDYDTTT where multiple Threonine residues flank Glutamic and Aspartic acid with a Tyrosine separating two Aspartic acid residues.
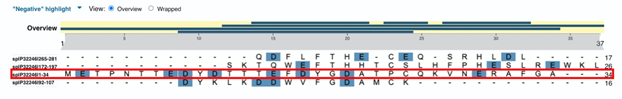
Figure 2 Results of alignment of human CCR1 extracellular domains. The first domain (1-34) in the third line. Negatively charged amino acids are highlighted.
The sequence TTEDYDTTT of the first extracellular domain of CCR1 has all the features that make it the most likely candidate for binding both Lysine and GalNAc at position 420 of Gc2 and GcMAF, respectively.
Lysine can interact with Tyrosine, Glutamic, and Aspartic acid through ionic interactions and hydrogen bonding. Lysine side chain has a primary amine group that can become positively charged at physiological pH. In contrast, Glutamic, and Aspartic acid have side chains that can become negatively charged at physiological pH. Therefore, the positively charged Lysine side chain can attract the negatively charged side chains of glutamate and aspartate, forming strong ionic interactions. While ionic interactions are prominent, hydrogen bonding can also occur between Lysine and the other amino acids. The amine group of Lysine can act as a hydrogen bond donor. Tyrosine's hydroxyl group on its side chain can act as a hydrogen bond acceptor. The carboxyl groups of Glutamic and Aspartic acid can also act as hydrogen bond acceptors. These hydrogen bonds, though weaker than ionic interactions, can contribute to stabilizing the ligand-receptor complex. While Tyrosine can't participate in ionic interactions like Lysine, it can still form hydrogen bonds with Lysine's amine group. The concomitant presence of ionic interactions with Glutamic and Aspartic acid can significantly increase the strength and specificity of the interaction between these amino acids.
In addition, Nabeshima et al.1 described the interaction of Thr418 of GcMAF and Gc2 with the receptor. The presence of multiple Threonine residues flanking the negatively charged amino acids in the sequence TTEDYDTTT of the first extracellular domain of CCR1 may be responsible for the interactions described by Nabeshima et al.1 Threonine residues facing each other on different proteins can interact with each other via hydrogen bonding and hydrophobic interactions. Each Threonine has a hydroxyl group on its side chain, which can act as both a hydrogen bond donor and acceptor. These hydroxyl groups can form hydrogen bonds with each other, creating a weak but stabilizing interaction between the two proteins. While not as strong as hydrogen bonding, weak hydrophobic interactions might occur. The side chain of Threonine has a methyl group; if the two methyl groups from the facing Threonine residues are positioned favorably, they experience attractive forces due to their hydrophobic nature.
The sequence TTEDYDTTT of the first extracellular domain of CCR1 also has all the features required to bind GalNAc. GalNAc can interact with Glutamic and Aspartic acid although the interaction wouldn't involve electrical charge like with a positively charged amino acid such as Lysine. Hydrogen bonding is the most likely type of interaction between GalNAc and Glutamic and Aspartic acid. GalNAc has hydroxyl groups on its sugar ring that can act as hydrogen bond donors whereas Glutamic and Aspartic acid both have carboxyl groups on their side chains that can act as hydrogen bond acceptors. In addition, GalNAc can interact with the Tyrosine that is flanked by Aspartic acid in the sequence TTEDYDTTT. Both Tyrosine and GalNAc have functional groups that can participate in hydrogen bonding. Tyrosine has a hydroxyl group on its side chain, which can act as a hydrogen bond donor. GalNAc has hydroxyl and carbonyl groups, which can act as hydrogen bond donors and acceptors, respectively. These interactions can occur between the side chain of Tyrosine and the hydroxyl or carbonyl groups of GalNAc, stabilizing a complex between the two molecules. The aromatic ring of Tyrosine can interact with the sugar ring of GalNAc through aromatic stacking interactions. These interactions can contribute to the overall stability of the complex. While both Tyrosine and GalNAc have hydrophilic groups, they also have some hydrophobic portions. Depending on the environment, weak hydrophobic interactions might occur between non-polar regions of the two molecules.
Although the literature on GcMAF dates back to 1994,17 studies on its putative receptor are more recent. In 2013, the potential interaction between the vitamin D receptor (VDR) and GcMAF based on their structural features was described by Thyer et al.10 The authors hypothesized that the C-terminal 23 hydrophobic amino acids of VDR, located on the cytoplasmic side of the plasma membrane, interacted with the N-terminal 23 hydrophobic amino acids of GcMAF, positioned on the extracellular side. This interaction could potentially create a binding pocket for 1,25(OH)2D3 and oleic acid sandwiched between the two proteins. Such an interaction between GcMAF and VDR was proposed on the basis of previous observation that the response of human peripheral blood mononuclear cells to GcMAF was higher in cells from donors whose BsmI VDR polymorphism was the one that showed the highest response to 1,25(OH)2D3 and paricalcitol, a non-hypercalcemic vitamin D analogue.18 Even though this hypothesis has not been disproven and might still be valid to explain some features of GcMAF responses, nevertheless, other hypotheses that have been put forward more recently merit consideration.
Nabeshima et al.1 propose that the GcMAF receptor is able to bind both the GcMAF characterized by GalNAc linked to Thr420 as well as the non-glycosylated Gc2, where Lysine at position 420 substitutes for GalNAc. In both cases, Thr418 participates in stabilizing the interaction. This hypothesis leads to the conclusion that there are at least two types of molecules with GcMAF activity, the bona fide GcMAF and Gc2. Therefore, the term GcMAF describes more of an activity rather than a single molecule. It is based on this hypothesis that the first extracellular domain of CCR1 was identified as the binding site for GcMAF and Gc2. However, if the term GcMAF might encompass an activity more than represent a single, well-defined molecule, then there are other molecules that have GcMAF activity, including sulfated polysaccharides such as chondroitin sulfate,19 and fucoidan.20 Supporting this hypothesis, Pacini et al.2 observed that heparin inhibits the effects of GcMAF on human peripheral blood mononuclear cells. This inhibition might be due to competition for receptor binding, as Singh et al.21 demonstrated that heparin can bind to CCR1, and this binding is influenced by the sulfation pattern of heparin.
Another recent hypothesis concerning the GcMAF receptor comes from the article by Kirikovich et al.22 who demonstrated that, in a cell-free environment, GcMAF can interact with the protein encoded by the CLEC10A gene, a C-type lectin domain family 10 member A that is a single-pass type II membrane protein also known as Macrophage lectin 2. Although these results merit consideration since GalNAc is known to bind to this protein, it is worth noting that Macrophage lectin 2 is not listed among the interactors of human Gc protein and is difficult to envisage a role for this protein in angiogenesis, autism, or neuroprotection, conditions where GcMAF has proven effective. It is also possible that the interaction observed by Kirikovich et al.22 might be due to the "sticky" nature of Gc protein, a protein whose function is, among others, to bind actin as an actin scavenger.23 In addition, the mechanism proposed by Kirikovich et al.22 does not take into consideration the observation by Nabeshima et al.1 that Gc2 has GcMAF activity despite the absence of GalNAc. However, it is also possible that GcMAF binds different receptors either on the same cell or in different cell types. Given that GcMAF shows a number of biological effects that go well beyond activation of macrophages, this type of pleiotropy and redundancy cannot be ruled out.
In a paradigm shift, radiation therapy is now understood to extend beyond local tumor control by inducing a systemic, or abscopal, effect on distant, untreated tumors. This newfound appreciation for radiation therapy's ability to trigger an immune response has fueled the exploration of combining radiation therapy with immunotherapy (radiation-immunotherapy). However, several key questions remain, including the precise mechanisms of radiation therapy-immune system interaction, optimal radiation-immunotherapy treatment schedules, strategies to enhance the abscopal effect, and methods to overcome radiation-immunotherapy resistance. The concept of the "radscopal effect," which utilizes low-dose radiation to reprogram the tumor microenvironment, presents a potential avenue to amplify the abscopal effect and overcome radiation-immunotherapy resistance. Collectively, these findings suggest that radiation therapy can be harnessed as a potent trigger of systemic antitumor immunity. When combined with immunotherapy, radiation therapy has the potential to become a cornerstone of curative and systemic treatment regimens for cancer patients (for rev. on radiation-immunotherapy, please see Zhang et al.24).
In the context of immunotherapy, GcMAF plays an unique role because of its multifaceted activities that target tumors from a variety of angles as reviewed in Ruggiero et al.,3 and Saburi et al.4 The ongoing exploration of the GcMAF receptor holds significant promise for understanding its potential role in radiation-immunotherapy. The identification of the first extracellular domain of CCR1 as a binding site for both bona fide GcMAF and its unglycosylated variant, Gc2, suggests a broader concept of GcMAF activity beyond a single molecule. This opens doors to exploring the therapeutic potential of other molecules like sulfated polysaccharides that might share similar binding capabilities. Overall, the search for the GcMAF receptor sheds light on the intricate mechanisms of GcMAF activity. Future research focused on these recently identified interactions could pave the way for exploring the potential of GcMAF, as an immunomodulatory therapy to enhance the effectiveness of radiation therapy in cancer treatment.
The author acknowledges the great work of Dr. Aldo Ruggiero, MD (1923-2006), pioneer of radiology in Prato, Italy, founder of the Studio Radiologico Ruggiero, source of boundless inspiration for this and many other scientific articles.
The author declares no conflict of interest in regard to the specific topics treated in this study.
This article is original and contains material that has not been submitted or published in any scientific journal. A preprint of the first submission of this article had been posted in a multidisciplinary preprint platform (Ruggiero, M. GcMAF in Radiation Therapy: Identification and Molecular Characterization of the Human GcMAF Receptor. Preprints 2024, 2024060455. https://doi.org/10.20944/preprints202406.0455.v2)
Not applicable.
Not applicable.
The author did not receive any funding for this study
No information in this paper is intended or implied to be a substitute for professional medical advice, diagnosis or treatment.

©2024 Ruggiero. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.