International Journal of
eISSN: 2574-8084


Case Series Volume 11 Issue 4
1Icon Cancer Centre, 1-3 Macarthur Ave, Revesby, NSW, 2022, Australia
2Icon Cancer Centre, 41 Williams St, Gosford, NSW, 2250, Australia
3Libby Paton Consulting, PO Box 347, Annandale, NSW, 2038, Australia
Correspondence: Professor Gerald Blaise Fogarty, Icon Cancer Centre, 1-3 Macarthur Ave, Revesby, NSW 2022, Australia
Received: July 15, 2024 | Published: August 1, 2024
Citation: Fogarty GB, Cheers S, Pritchard A, et al. Experiences with Volumetric Modulated Arc Therapy (VMAT) for treating symptomatic peripheral joint osteoarthritis (OA) with Low Dose Radiotherapy (LDRT) – a case series. Int J Radiol Radiat Ther. 2024;11(4):92-98. DOI: 10.15406/ijrrt.2024.11.00393
Introduction: Peripheral joint osteoarthritis (OA) in Australia is the third leading cause of life-years lost. Implant surgery is a great help, but patients who are waiting or are inoperable suffer. Low Dose Radiotherapy (LDRT) is well established in some countries. Three-dimensional conformal radiotherapy (3DCRT) is recommended. However, some departments may only have Volumetric Modulated Arc Therapy (VMAT). We present our experiences treating ten volumes of symptomatic OA treated with VMAT in nine consecutive patients to two months post-LDRT.
Methods: The hypothesis was that LDRT using VMAT was feasible and effective at two months. Patient response data was collected prospectively. The Visual Analog Scale Pain (VASP) for pain was used. A Visual Analog Scale Mobility (VASM) was created for mobility. Bones within radiation oncologists (RO) skin marks around the joint were auto-contoured for clinical target volume (CTV) with 2 mm expansion to planning target volume (PTV). Treatment was titrated to clinical response. Phase 1 was 3 Gray (Gy) in 6 fractions at 2-3 fractions per week. There was RO review at 2 months post-phase 1 for consideration of phase 2. If no response, then phase 2 was 6 Gy in 6 fractions, if a partial response (PR), then a repeat of phase 1. A third phase similar to phase 2 was possible. RO follow-up was done 2 months after the last phase.
Results: Nine consecutive patients, eight males and one female, average age of 69 years (60-84), with ten volumes of symptomatic OA were treated. Joints were three single knees, two cases of both knees, three cases of both hands, one hip and one carpometacarpal joint. They had suffered from OA for an average of eight years (1-20). All were on at least one systemic therapy. All were using some sort of local therapy or device. Eight had a PR to phase 1 and proceeded to phase 2. Two had an equivocal response. No patient had a third phase. Average VASP pain scores fell from baseline of 7.4 to 3.6 after phase 1, and to 2.3 after phase 2. Average VASM immobility scores fell from baseline of 6.9 to 4.4 after phase 1 and to 3.3 after phase 2.
Conclusion: In this small Australian cohort LDRT using VMAT is feasible and clinically effective when measured at 2 months. More study is needed.
Keywords: intensity-modulated, radiotherapy, volumetric modulated arc therapy, osteoarthritis, inflammation, peripheral joint, case study, Australia
In Australia, OA is the third leading cause of life-years lost due to disability and is greatest cause of pain and disability among the elderly.1 The guideline development group of the UK’s National Collaborating Centre for Chronic Conditions for Osteoarthritis (OA) considered a clinician’s working diagnosis of peripheral joint osteoarthritis to be the following: age 45 years old and over, persistent joint pain that is worse with use, and morning stiffness lasting no more than half an hour.2
Treatment is applied with a holistic approach. Lifestyle factors such as increased exercise and avoiding obesity can help. Nonsteroidal anti-inflammatory drugs (NSAIDS) are used but also carry risks including cardiovascular (CV) events, gastrointestinal bleeds, and chronic or acute renal failure.3 About 25% of all patients will not respond or lose their responsiveness to NSAIDs over time.4 OA has been associated with increased risk of developing CV by 50% and is a major cost to the United States health budget.5
Treatment aimed at specific joints include intra-articular NSAIDs and corticosteroids. Surgical intervention such as joint lavage, debridement, synovectomy, and eventually prosthetic implant replacement are common, but are expensive and carry risks of bleeding and infection. Those considered too young or too frail for an implant may have to endure years of pain and immobility, leading to decreased quality of life (QoL).
Low Dose Radiotherapy (LDRT) for treating symptomatic OA is based on a solid radiobiological platform.6-8 LDRT is well established in some countries9 with national guidelines10 and a large throughput of patients each year.11 Axial joints are avoided to protect bone marrow. Planning guidelines have been developed using three-dimensional conformal radiotherapy (3DCRT).12 3DCRT has been recommended as the radiotherapy (RT) modality of choice as it is available on most linear accelerators (LAs). The extra volume of normal tissue irradiated compared with using intensity modulated radiotherapy (IMRT) and similar modalities like Volumetric Modulated Arc Therapy (VMAT)13 is at a very small increased risk of radiation induced malignancy (RIM). However, some departments may only have LAs that deliver IMRT/VMAT. We present our experiences treating ten volumes of symptomatic OA treated with VMAT in 9 consecutive patients.
The hypothesis of this study was that LDRT using VMAT was feasible and clinically effective measured at two months post LDRT in the Australian context. The aim was to treat 10 volumes of symptomatic OA. Patients were selected according to the following criteria as detailed in Table 1.
|
# |
Criteria Description |
|
1 |
Age over 60 |
|
2 |
At least one symptomatic joint; joint could be a large (e.g. knee, hip) or small (carpometacarpal (CMC) joint of thumb) or a collection of joints (e.g. whole hands) |
|
3 |
OA pain for at least 6 months |
|
4 |
OA pain was limiting of normal activity |
|
5 |
There is also limited mobility of the joint(s) in question |
|
6 |
On at least intermittent oral therapy (e.g. NSAIDs) for pain from that joint |
|
7 |
Radiological confirmation of OA in the target joint(s) |
|
8 |
Able to receive VMAT for the joint(s) in question |
|
9 |
Able to fill out a pain and mobility questionnaire |
Table 1 Eligible patient selection criteria
Protocol
Prior to treatment, the radiation oncologist (RO) took a history and performed physical examination. Extra questions specific to OA were asked and recorded. These questions are detailed in supplementary material Appendix A. The doctor and patient went through this form together to ensure clarity. Other reasons for pain and immobility were enquired about (e.g. trauma). Apart from taking a rheumatological history no other causes of arthritis (e.g. gout, psoriasis) were excluded by investigation.
The Visual Analog Scale Pain (VASP) for pain was used among other questions. A Visual Analog Scale Mobility (VASM) for mobility like the VASP was created. Physical examination looking for features of OA such as joint effusion, crepitus, deformity was done. Investigations including radiological confirmation of OA were sought. Consent for LDRT for OA was obtained, as was permission to use de-identified data for reporting and publication. Nurse education was also performed. All was documented as usual.
For RT planning, the RO marked on skin the radiation field edges of the volume to be treated. These marks could be upper, lower, medial and lateral and any other limits. These were in general aimed to cover all the synovium associated with that joint volume. (Figure 1) These marks were interpreted by the planning radiation therapists (RThers) to be one centimetre (cm) outside limits of the proposed clinical target volume (CTV) and eight millimeters (mm) outside of the planning target volume (PTV).14 (Figure 1A)
Figure 1 Clinical mark ups of volumes to be treated.
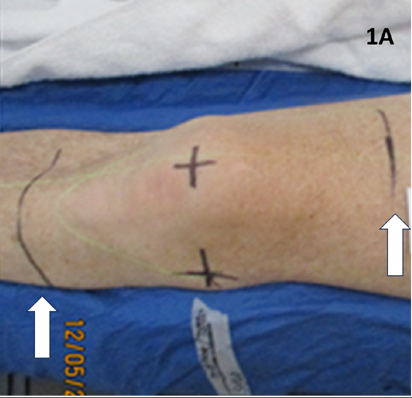
Figure 1A Clinical mark up of a knee. White arrows show RO marks that will determine auto-contoured CTV and PTV. A large volume has been marked as the CTV needs to cover the whole synovium.
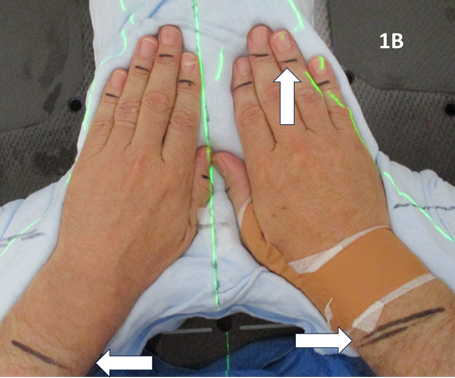
Figure 1B Clinical mark up of both hands. White horizontal arrows show RO marks that will determine auto contoured CTV and PTV. This large volume covers all the wrist and hand synovial membranes. The white vertical arrows show the nail beds excluded.
RThers then record these skin marks with photos and templates so they could be wired and captured at the simulation planning computed tomography (CT) scan. The RThers then positioned the patient on the simulator to enable VMAT with appropriate immobilisation devices to ensure reproducibility and patient comfort during treatment as per departmental protocol. CT scanning with 2 mm slice thickness was performed to adequately cover the appropriate volume for contouring and dosimetry. The RThers then auto-contoured the bones in the volume within the RO skin marks. The bones comprised the CTV. The CTV in the superior – inferior direction was minus one cm from the skin marks. The PTV was a 2 mm expansion on the CTV.
These volumes were then approved by the RO. The target volume was intended to be the joint including synovium with prescription to cover the PTV with 95% of the prescription dose. (Figure 2)
Figure 2 Contouring and dosimetry.
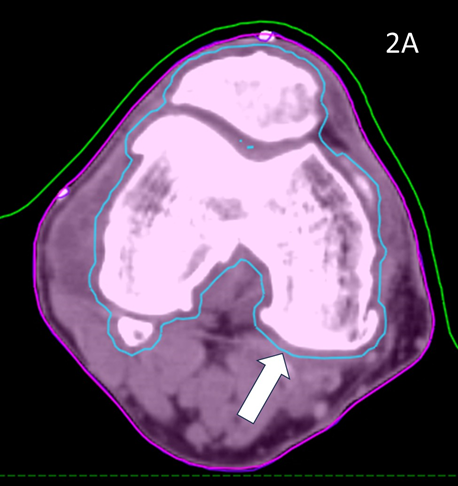
Figure 2A Axial planning CT slice through zero plane of knee in Figure 1A showing PTV (blue volume indicated by white arrow) as 2 mm expansion of CTV which was an auto contour of the bone.
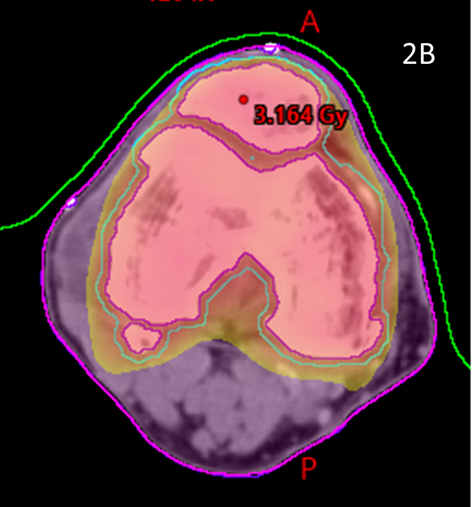
Figure 2B Axial planning CT slice through zero plane showing dosimetry as colour wash set at 2.5Gy level. Notice excellent conformity to PTV and homogeneity within the PTV and sparing of soft tissues that may not have been spared by a 3DCRT approach.
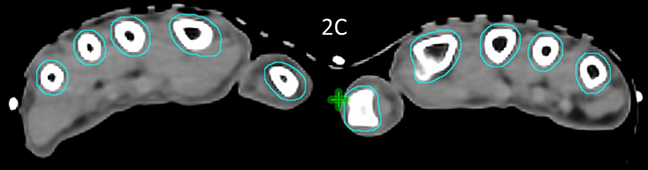
Figure 2C Axial planning CT slice through zero plane of hands in Figure 1B showing PTV (blue volume) as 2 mm expansion of CTV which was an auto contour of the bone.
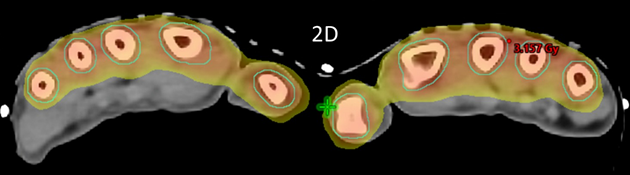
Figure 2D Axial planning CT slice through zero plane showing dosimetry as colour wash set at 2.5Gy level. Similar to Figure 2B, notice excellent conformity to PTV and homogeneity within the PTV while sparing the palms.
The RT dose prescription was suggested by international leaders (Dr Richard Shaffer, personal communication)15 in the field and this prescription has now become standard in many places. The dose was planned to be given in up to three phases. The dose per phase and number of phases is given according to clinical response.
Phase 1 is three Gray (Gy) given in six fractions (0.5 Gy per fraction) given at two to three fractions per week, the frequency depending on patient preference. There was a RO to review at two months post phase 1 for consideration of phase 2. The questions made at baseline were repeated and answers documented as detailed in supplementary material Appendix A. If no response, then phase 2 is prescribed as 6 Gy in 6 fractions (1 Gy per fraction). If a partial response, then phase 2 is 3 Gy in 6 fractions (0.5 Gy per fraction) given at 2-3 fractions per week. The RO approves the dosimetry prior to each phase. As there is no acute toxicity at these doses, no routine on-treatment reviews (OTRs) need to be scheduled. If there are concerns at patient or staff level an extraordinary OTR can usually easily be arranged.
There is then a further RO to review at two months post-phase 2 for consideration of phase 3. A further phase 3 can be offered as 6 Gy in 6 fractions (1 Gy per fraction) if still no response, or if still partial and needing or desirous of more treatment, 3 Gy in 6 fractions could be given.
The maximum dose for any patient was therefore to be 3+6+6 = 15Gy in a very fractionated manner (total of 18 fractions over 4-6 months) so no organ at risk structures (OARs) needed to be contoured. Replanning CT and contouring between the phases was not required as the bone volume did not change. If there was a change due to other factors between phases due to, for example, weight loss or peripheral lymphoedema, this could be caught on the first CBCT prior to retreatment and replanning done.
Follow-up assessment was performed by the RO 2 months after the last phase. Data forms similar to Appendix A were filled out at each time point prospectively.
Patient characteristics
Nine consecutive patients who satisfied the selection criteria with ten volumes of symptomatic OA to treat were included. Their baseline characteristics are summarised in Table 2A. Briefly, there were eight males and one female. Average age was 69 years (60-84). Of the 10 areas, three were treated on a True Beam LA (Varian, Palo Alto) while seven were treated on a Halcyon (Varian, Palo Alto). Referrals were written by seven General Practitioners (GPs) and two specialists, an immunologist and an orthopaedic surgeon.
|
No |
Age/ Gender |
How ref |
Body part |
Yrs |
Time of Worse Symp |
Systemic |
Local |
Implant |
Base Pain |
Base Mob |
|
1 |
63M TB |
WoM |
Knee |
5 |
Weather |
NSAID |
Cream |
Waiting |
7 |
7 |
|
2 |
64M TB |
PP |
Hands |
10 |
Mornings |
NSAID |
Ice packs |
NA |
9 |
8 |
|
3 |
67F TB |
WoM |
Hip |
6 |
Weather |
paracet |
Hot water |
Declined |
10 |
8 |
|
4 |
84M Hal |
Ref |
Knee |
5 |
Wt bear |
NSAID |
nil |
Too frail |
7 |
Can’t say |
|
5 |
61M Hal |
WoM |
Knee |
5 |
Wake night |
NSAID |
Hot water |
Waiting |
7 |
9 |
|
6 |
60M Hal |
WoM |
RCPC |
1 |
On weight bear |
NSAID |
Brace |
Considering |
7 |
5 |
|
7 |
81M Hal |
Ref |
Hands |
5 |
Night time |
Opiates nocte panadol |
Failed cannabis |
NA |
8 |
10 |
|
8 |
66M Hal |
Ref |
Knees |
20 |
Weather, up stairs |
Opiates NSAID |
Physio |
Too frail |
7 |
7 |
|
9 |
78M Hal |
Ref |
Knees |
20 |
All the time |
Opiates |
Scooter |
Too frail |
7 |
9 |
|
10 |
64M Hal |
WoM |
Hands |
3 |
With use |
NSAID |
Ice water |
NA |
5 |
6 |
|
Av |
69 |
- |
- |
8 |
- |
- |
- |
- |
7.4 |
6.9 |
Table 2a Patient characteristics at baseline
Legends - No, number; Ref - referred by a referrer; WoM – word of mouth by staff; PP - a previous patient with another diagnosis; M, male; F, female; TB, True Beam; Hal, Halcyon; RCPC, Right carpometacarpal joint; Yrs, Years; Wt, Weight; Sympt, symptom; NSAID, non-steroidal anti-inflammatory drug; paracet, paracetamol; NA, not applicable; mob, Mobility; Av, Averages.
Volumes treated were three single knees, two cases of both knees, three cases of both hands, one hip and one carpometacarpal joint. They had suffered pain and immobility in these joints for an average of eight years (1-20). Four had the worst symptoms when they were using the joint involved but six had worst symptoms when they were not using the joint involved, for example waking up at night in pain or when there was a change in the weather. All were on at least one systemic therapy, some on multiple and some on regular opiates. All used some sort of local therapy, for example, a topical cream, ice packs, hot water, a brace, regular physiotherapy or a mobility scooter to get around without pain. Two were awaiting implants, three could not have any implants as their whole hands were painful and one had declined an implant, whereas three were too frail for major surgery. There was no rescanning needed. No extraordinary OTRs were necessary.
Before any radiotherapy the average pain score was 7.4 (5-10) out of 10, and the average mobility score was 6.9 (5-10).
Insert Table 2A here. Patient characteristics at baseline
Patient response to treatment was recorded at 2 months after each phase. These are detailed in table 2B. Eight volumes had a partial response to phase 1 on the VASP and VASM. They also decreased their use of drugs and non-drug aids (for example, need for soaking hands in cold water, stopped use of ice packs, did not wake in pain etc). For those who had radiation of pain, the radiation decreased. There was an increase in QoL, one could now lift his grandchild, another could go back to work, one with a bad knee went back to playing soccer, another could now grip golf clubs and had returned to play, others could walk or go downstairs without pain. One said he was sleeping better and less angry.
|
No |
Pain |
Mob |
Result |
Ph 2 given |
Pain |
Mob |
Total change |
Changes |
|
1 |
4 |
6 |
PR |
Yes |
2 |
4.5 |
P 5; M 2.5 |
No more drugs, Can walk without pain. |
|
2 |
3 |
5 |
PR |
Yes |
2 |
2 |
P7; M6 |
Back at work, Can lift grandchild, Less irritable, No ice packs, No wake in pain. |
|
3 |
6 |
6 |
PR |
Yes |
4 |
5 |
P6; M3 |
Stairs now ok, No more hot water bottle, No radiation to knee. |
|
4 |
5 |
5 |
PR |
Yes |
Can’t say |
Can’t say |
Can’t say |
Wife says much better. |
|
5 |
0 |
3 |
PR |
Yes |
1 |
3 |
P4; M6 |
Playing soccer. |
|
6 |
4 |
4 |
PR |
Yes |
4 |
4 |
P3; M1 |
To wrist implant. |
|
7 |
3 |
3 |
PR |
Yes |
1 |
1 |
P7; M9 |
After phase 1 was back to golf, Can open jars, No need for cutlery grips. |
|
8 |
8 |
5 |
Stable |
No |
|
|
|
After phase 1 can get out of house. |
|
9 |
Can’t say |
Can’t say |
Can’t say |
Yes |
Can’t say |
Can’t say |
Can’t say |
Probably no difference, foot ulcer was a distraction. |
|
10 |
1 |
3 |
PR |
No |
- |
- |
P4, M3 |
- |
Table 2b Patient characteristics two months post phase 1 and 2
Legends - No, number; Yrs, Years; Ph, Phase; PR, partial response; P, pain; M, mob, Mobility.
These eight proceeded to phase 2 with a further 3 Gy in 6 fractions. Of the eight treated, two could not reliably give a figure on the VASP or VASM. Of those who could, there was a further reduction in pain and improvement in mobility, but not to the same amount after phase 1. Average VASP pain scores fell from baseline of 7.4 to 3.6 after phase 1 and 2.3 after phase 2. Average VASM immobility scores fell from baseline of 6.9 to 4.4 after phase 1 and 3.3 after phase 2.
Two had an equivocal response to phase 1. One said he could not tell whether the pain had improved even though his wife said he had improved in terms of less pain complaints, less use of medication and better mobility and mood. He went onto phase 2. One patient (8) had increase in pain from 7 at baseline to 8 after phase 1 and a decrease in immobility from 7 to 5 and declined phase 2. He said that his OA normally got worse in the colder weather, and this is probably the reason why the pain increased as winter deepened. This patient declined the second phase. Another (10) did not have the phase 2, He had bilateral hands treated and had such a good response (pain from 5 to 1, mobility from 6 to 3) that he thought he would leave the second phase to when he needed it more.
No patient received a dose of 6 Gy in 6 fractions, the third phase. One proceeded to an implant two months after phase 2 with what he thought was no relief from the radiotherapy. His initial pain score was 7 and mobility score was five, his final pain score was 4 and mobility of four. (Table 2B)
Insert Table 2B here - Patient characteristics two months post phase 1 and 2.
Response of pain and mobility to LDRT
Of those who could give a reliable score for pain and mobility the following diagrams were made. Figure 3A is for pain response, figure 3B for mobility. (Figure 3)
Figure 3 Response over time
The hypothesis of this study was that LDRT using VMAT was feasible and clinically effective in the Australian context as measured at two months post-LDRT. LDRT was feasible. The protocol as described above was workable between the radiation craft groups. Because of the low dose of radiotherapy needed, there was no need to involve the nurses or the ROs in OTRs. LDRT was effective in terms of decreasing pain and immobility according to the measurements used and measured at 2 months post phase 2. Patient response data was collected prospectively. Average VASP pain scores fell from baseline of 7.4 to 3.6 after phase 1 and to 2.3 after phase 2. Patients in general seemed to understand our novel VASM device. Average VASM immobility scores fell from baseline of 6.9 to 4.4 after phase 1 and 3.3 after phase 2.
There was less improvement after the second phase in both pain and mobility. Some have advocated to just have one phase. However, the decrease in improvement could just reflect the subjective nature of how these reporting systems work. It would be unusual to have a greater improvement after phase 2 than phase 1. Perhaps a better way to assess effect of phase 2 would be to ask them to grade pain prior to phase 2 as a ten.
These results conflict with randomised trials16-18 which showed no significant improvement. In these trials there was some excellent design characteristics such as sham radiotherapy. However, the protocols may not have been appropriate, with one Gy fractions being given, only one phase being delivered, and problems with accrual leading to early trial closure.
VMAT does deliver a lower integral dose13 than 3DCRT but at this low dose this is probably not relevant in reducing RIM risk. This is always a concern when irradiating benign diseases. One study estimated the risk of fatal tumour induction in patients treated with RT for various benign conditions. In the study, the estimated lifetime risk for an induced fatal tumour for a patient receiving LDRT with total dose of 6 Gy for knee OA at the age of 25, 50, and 70 was 2 in 1000, 0.7 in 1000, and 0.3 in 1000 patients, respectively, when assuming an estimated effective dose of 13 mSv (which, of note, is an effective dose similar to an abdominopelvic computed tomography [CT] scan).19 Although very rare, RIM does need to be raise at the consent stage. Patients in our small cohort were not put off RT by this risk. We used VMAT as in most cases this was the only modality available.
Some more anecdotal experiences of the investigators of this small cohort were the following.
Referrals
Referrals were really promoted by word of mouth from patients and even departmental staff. Four were referred by long-standing GP referrers to the practise, usually who heard about this study from the author. One patient was an Uber driver who incidentally complained of a sore knee while driving and was invited onto the study; another was a previous patient with another diagnosis. One patient had two volumes treated metachronously, the second after a successful first volume.
Most were referred by GPs. This makes sense as usually an orthopaedic surgeon would see a patient once. From there the patients can go straight to surgery for an implant or operation is not offered as they are too young or too infirm. The latter two groups will then see their GP regularly for regular supplies of pain medication. It's the GPs that manage the pain and bear the risk of prescribing these drugs and who would be open to finding a better solution. There was no active advertising to specialists for this treatment and there may not need to be, given that most patients essentially self-referred.
Inflammatory over degenerative response
Those with more inflammatory symptoms (e.g. more issues in the morning with pain and stiffness, waking with pain, joint swelling and change in symptoms with the weather) as compared with degenerative pain (e.g. pain only when using the index joint in a weight bearing position) seemed to respond sooner and better to LDRT. Those with more degenerative symptoms like pain on a specific movement did not seem to do as well. Response was seen in some after the first fraction, other not until assessment for the next phase.
Degree of suffering
Another experience was just how much these patients suffer. All of them had significantly changed their lifestyle to cope with the pain and immobility of OA. A further experience was that LDRT did not relieve all their pain entirely but certainly decreased the pain and increased the mobility enough for them to have significant increases in their quality of life which included their relationships. They were not so much interested in getting rid of all their pain but having just enough pain and immobility relief to allow them to return to more normal function.
Ability to cope with the data forms
There were two groups of patients. Those who were waiting for an implant who tended to be younger and healthier and had been told by their surgeons to come back later. The other group were older and frailer and beyond surgery. The latter found that the VASP and VASM and the questions in Appendix A more challenging. Patient 4 and Patient 9 could not commit to a number after treatments. Filling out the forms in the context of a structured interview led to better compliance. Others had to come to a compromise. In general, those with more inflammatory symptoms found it more difficult to settle on a score due to the fluctuating nature of pain and mobility with the day and the temperature. These experiences may influence which patients would be better to enrol in a proper prospective study and how to collect the data.
Individual anecdotal experiences
Patient 5 had treatment of a symptomatic left knee. The planning scan showed an effusion measuring 43.0cm3. Three months later the final CBCT of phase one showed a volume of 37.7cm3. A further three months later the final CBCT of phase two showed a volume of 33cm3, an overall decrease of 25%, even though he had increased activity by restarting playing soccer in that time. The reduction from planning CT to the final CBCT is shown in figure is shown in Figure 4. The effusion decreased in volume even though the dose was prescribed to bone. The reduction in the effusion associated with a reduction in symptoms and a better quality QoL points to this effect being more than just placebo as some have opined.
Figure 4 Decrease in size of knee effusion with two phases of 3 Gy in 6 fractions over three months.
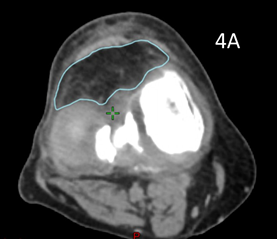
Figure 4A Axial planning CT scan through the knee showing effusion coloured in light blue measuring 43.0cm3.
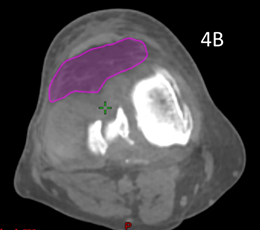
Figure 4B Axial planning CBCT scan taken during the last fraction of Phase 2 through the knee with effusion coloured in purple showing a volume of 33cm3.
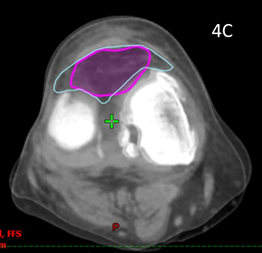
Figure 4C Fusion of the above two scans showing significant shrinkage of 25% of the effusion from baseline to end of phase 2.
Patient 9 had LDRT as he was too frail for bilateral knee implants. He had a significant delay of 4 months between phase 1 and 2 due to extended hospitalisation for treatment for unrelated comorbid disease. The immobility caused by his illness and the delay may have impacted the efficacy of treatment. He could not reliably give a score for pain or mobility after baseline.
Our small series, perhaps one of the first in Australia, shows that LDRT using VMAT is feasible and effective at 2 months according to the instruments used for measurement of pain and immobility. At this stage, radiotherapy is not used routinely for OA in Australia. This treatment was popular, and patients readily came, being attracted by word of mouth by other patients and staff. There is certainly a need for these patients suffering from OA for more effective treatments, particularly those who are waiting for an implant or too frail to be operated on. More study is needed.
The authors would like to acknowledge the participating patients and their referring doctors; Drs Kate Jin, Mhimid Farhad, Donna Burgess, John James, Yogesh Kalra, Stephen Nicol, Karl Baumgart, John Prossor, David Bradshaw. Thanks to Jenson O'Leary for graphics assistance with Figure 3. Thanks to Dr Richard Shaffer for his help with the protocol.
Gerald Fogarty: Shareholder Genesis Care 2008—current; Advisory board 2020-3 La Roche- Posay Pty Ltd.; Advisory board 2017 Merck Serono Australia Pty Ltd.; Advisory board 2020 Sanofi-Aventis Australia Pty Ltd.; Grant recipient 2018, 2019, 2020, 2021 Merck Serono Australia Pty Ltd. and Grant recipient 2021, 2022 Sanofi-Aventis Australia Pty Ltd. The remaining authors declare no other conflicts of interest.

©2024 Fogarty, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.