International Journal of
eISSN: 2574-8084


Case Series Volume 11 Issue 1
1Icon Cancer Centre, 1-3 Macarthur Ave, Revesby, NSW 2022, Australia
2Faculty of Science, University of Technology Sydney, 15 Broadway, Ultimo, NSW 2007, Australia
3Monash University, Faculty of Medicine, Nursing and Health Sciences, 27 Rainforest Walk, Clayton VIC 3168, Australia
4School of Medicine, The University of Notre Dame Australia, 160 Oxford St, Darlinghurst, NSW 2010, Australia
552 Meredith Street, Bankstown, NSW 2200, Australia
Correspondence: Gerald B. Fogarty, Icon Cancer Centre, Revesby, New South Wales 2122, Australia, Tel +61 (2) 87222800, Fax +61 (2) 87222801
Received: March 08, 2024 | Published: March 20, 2024
Citation: Fogarty GB, Maunder R, Nguyen K, et al. Definitive superficial radiotherapy for challenging skin cancers – a case series of experiences and learnings. Int J Radiol Radiat Ther. 2024;11(1):8-14. DOI: 10.15406/ijrrt.2024.11.00377
Definitive radiotherapy (RT) for cancer treatment offers tissue conservation. Superficial radiotherapy (SXRT) is an excellent modality for treating selected skin cancers. Through this case series, we aim to assist clinicians new to SXRT to deliver high quality care. We outline our experience in treating cancers of the lips, lower legs and ears, and discuss important planning and patient management techniques such as appropriate shielding, tumour radiation sensitivity, adequate nutrition, surveillance of regional lymph nodes, acute toxicities and patient follow-up. We highlight our experience with dose titration in response to the acute effects of RT on the cancer, and we also consider chyle leaks on the lower legs and the use of adaptive split course radiotherapy (ASCRT). Finally, we discuss the need for regular patient reviews as SXRT may not remain the most appropriate treatment modality if the lesion changes during the therapeutic course.
Keywords: radiotherapy, skin cancers, case report, Australia
BCC, basal cell carcinoma; cSCC, cutaneous squamous cell carcinoma; RT, definitive radiotherapy; SXRT, superficial radiotherapy; HVL, half value layer; LN, lymph node; PET, positron emission tomography; FNAB, fine needle aspiration biopsy; RBE, relative biological effectiveness; ASCRT, adaptive split course radiotherapy; MeV, mega electron volt
Keratinocyte cancers, or non-melanoma skin cancers such as basal cell carcinoma (BCC) and cutaneous squamous cell carcinoma (cSCC), are the most common cancers in Australia and the United States.1,2 Known risk factors for developing keratinocyte cancers include fair complexion, advancing age, immunosuppression, and chronic ultraviolet radiation.3
While surgery remains the main treatment modality for keratinocyte cancers, definitive radiotherapy (RT) has a significant role. RT is widely accepted as an alternative to surgery in patients who refuse surgery or are not good surgical candidates, and where cosmetic or functional outcome is a concern due to the benefit of tissue conservation.4
Among the RT modalities, superficial radiotherapy (SXRT) is one of the oldest, having been first developed over 100 years ago. It was an integral part of a dermatologist’s practice for many years until new surgical techniques gained popularity, which led to a decline in its use. More recently, SXRT is seeing a resurgence as advocates seek to re-establish its efficacy and benefits.3
SXRT is an excellent modality for treating appropriate types of keratinocyte cancers. In one large retrospective study involving 1715 histologically confirmed BCCs, SCCs and mixed lesions, a five-year overall recurrence rate of 5.0 percent was demonstrated.5 Furthermore, SXRT offers the practical advantage of being able to visualise the target tumour as it is not hidden under the bolus material usually required for megavoltage (MV) treatments to ensure therapeutic dose to skin.
In this paper, we present a summary of cases recently treated with SXRT to the lips, lower legs and ears with the aim that our experiences may help colleagues embarking on their own SXRT program.
Lips
SXRT is ideal for early lip cancer as planning can be undertaken with shielding in place. There is minimal penumbra, and patient set up is straightforward. We present three cases of lip cancers that were successfully treated to a complete response (CR) with SXRT.
Case 1
A fit 77-year-old Caucasian woman was referred for definitive SXRT for a T1N06 biopsy-proven ulcerated cSCC on the midline of the lower lip measuring 15 millimetres (mm) at its greatest extent and 0.4 mm deep on biopsy. On examination the lesion was thin, but all the lower lip was sun damaged with clinical evidence of extensive Bowen’s Disease. There were no signs of perineural invasion or lymphadenopathy. The decision was made to treat the whole lower lip.
The patient was treated with SXRT to 60 Gray (Gy) in 30 fractions five days a week using a beam with half value layer (HVL) of 1.9 mm aluminium (Al) and a generating energy of 100 kV. Set up included a lead shield that was inserted inside the lower lip to protect the lower jaw, teeth, and gums; the same shield in continuity was also placed outside the upper lip. This protected the upper lip from receiving any RT.
Shielding is an important consideration to protect healthy structures. Patients need to keep eating during treatment, and oral intake is aided by having at least one lip that does not suffer from the acute effects of RT. Adequate nutrition helps with weight preservation during treatment. Maintaining weight ensures that the surface contour of the treated area does not change, enabling the dose to be delivered as planned. Normal cells will also only repair the damage inflicted by the RT between fractions if they are well nourished. If not, the acute effects will be worse and patients may ask for a break or even cessation of RT, risking tumour recurrence (Figure 1A&1B).
Figure 1 Lower lip.
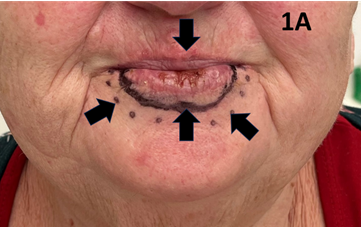
Figure 1A cSCC of the lower lip. There is a malignant ulcer amid field change this is typical of sun induced cancers. Arrow pointing vertically down shows the malignant ulcer. Arrow pointing vertically up shows a black solid line depicting the clinical target volume (CTV). Arrows diagonally up point to the SXRT field edge.
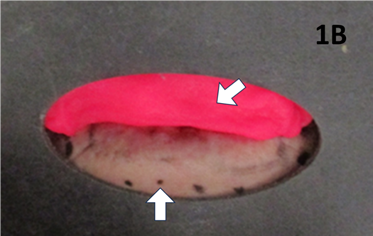
Figure 1B Lead equivalent outertemplate for SXRT treatment. Arrow pointing vertically up shows the lower field edge as seen in 1A. Diagonal arrow shows ‘superstuff bolus’, commonly known as ‘pink stuff’ (NL-Tec; Willeton, Western Australia) to ensure adequate dose to the lip’s surface.
The patient achieved a CR that was maintained at last follow-up (four months post treatment).
Case 2
An 83-year-old lady with significant comorbidities presented with a biopsy-proven exophytic T2N06 cSCC of the lower left lip (Figures 2A & 2B). She was treated with definitive SXRT using the same parameters as for Case 1. Only the lesion was treated. During treatment, a left submandibular lymph node (LN) became palpable (Figure 2C). A positron emission tomography (PET) scan revealed a solitary LN which was confirmed as SCC on fine needle aspiration biopsy (FNAB). Due to unrelated sickness, treatment was interrupted and delayed. The patient eventually finished the course and achieved a CR at the primary site but had a growing 5.0 centimetre (cm) left submandibular LN. Fortunately, this responded to the immunotherapy that was started immediately upon the completion of SXRT (Figure 2D). An important experience is that a LN can grow quite quickly even during treatment of the primary lesion.
Figure 2 Left lower lip.
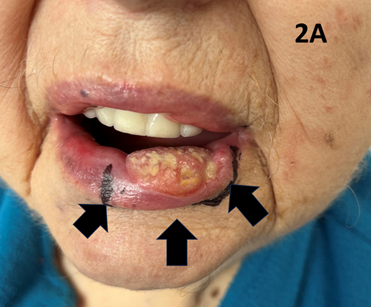
Figure 2A Large T2 cSCC of the left lower lip on presentation. Vertical arrow points to the tumour. Diagonal arrows show the SXRT field outline. There is no visual sign of any lymphadenopathy.
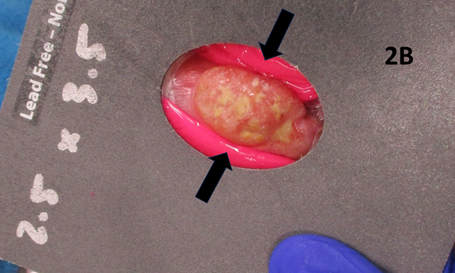
Figure 2B Treatment set up. Arrows show the bolus placement to ensure that the full dose is bought to the tumour’s surface.
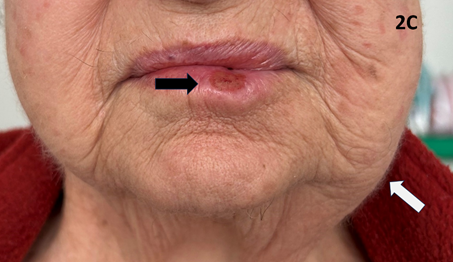
Figure 2C Black arrow points to the responding tumour during treatment. However, a left submandibular lymph node (LN) become symptomatic, visible, and palpable (white arrow) during an interrupted treatment. A PET scan revealed a solitary LN which was confirmed as cSCC on FNAB. This responded to immunotherapy.
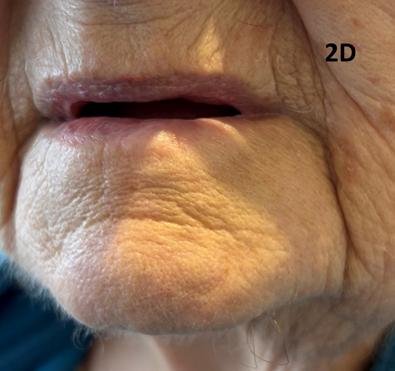
Figure 2D The treatment course was eventually finished, and the patient achieved a CR at the primary site. The lymph node disappeared with immunotherapy.
Case 3
A 55-year-old lady with a history of cannabis smoking presented with a rapidly growing exophytic lesion in the midline of the lower lip. A biopsy confirmed cSCC. The patient proceeded with SXRT to a dose of 60 Gy in 25 fractions with a similar quality beam as in Case 1. Only the lesion was treated, and the patient achieved a CR six weeks post treatment (Figures 3A & 3B). Our anecdotal experience is that exophytic skin lesions tend to be more radiation sensitive than endophytic ones. Although more research is needed, the rationale behind this observation is that exophytic lesions bring their blood supply from the dermis, resulting in enhanced oxygenation.7 As oxygen is a radiosensitizer, the relative biological effectiveness (RBE) is improved compared to anoxic conditions.8,9
Figure 3 Midline lower lip.
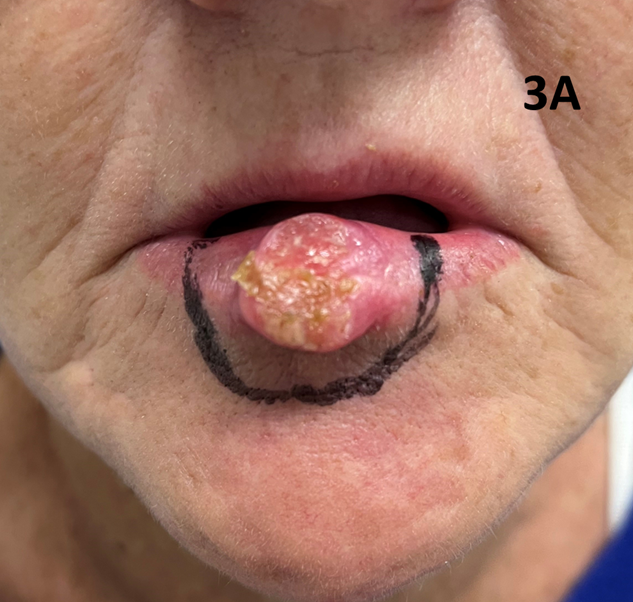
Figure 3A A large T2N0 SCC is clearly visible on the midline lower lip in a 55-year-old lady with a history of cannabis smoking. The black line marks the SXRT field edge. Only the lesion was treated. Note the normal appearance of the remaining lip outside the treatment field compared to Case 1.
Legs
Case 4
A 66-year-old lady presented with a recurrent locally advanced and symptomatic T2N06 well-differentiated cSCC on the right lateral foot. The patient was initially planned for definitive surgery to treat her cancer; however, she was a vasculopath with no palpable pulses in any arteries below the groins. As re-establishing vascular flow was deemed necessary before proceeding, the patient was first booked in for a bilateral femoral-popliteal bypass graft. Given that she had other comorbidities and was a high-risk surgical candidate, definitive SXRT to the foot lesion was considered a much safer and more viable treatment option (Figure 4A & 4B).
Figure 4 Right lateral foot.
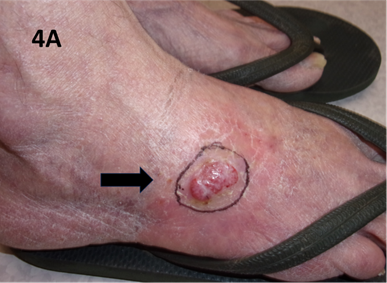
Figure 4A T2 N0 well-differentiated recurrent cSCC of the right lateral foot. The black arrow indicates the lesion and SXRT field.
The patient was treated with SXRT to a total dose of 55 Gy in 25 fractions using the same beam quality as mentioned in Case 1. Towards the end of treatment not enough acute toxicity was evident. Was this a result of hypoxia due to vascular insufficiency which, in turn, decreased the oxygen enhancement ratio (OER)? A three-fraction boost of 6.6 Gy in total was added. The patient went on to achieve a CR which was maintained one year later (Figure 4). Vascular surgery was cancelled.
An important learning is that dose titration based on the acute effects in surrounding normal tissue can help finesse the actual dose needed to sterilise the cancer.
Case 5
A well and independent 90-year-old woman had a 2.5 cm lesion arise on the lateral right ankle over a period of months. Her local doctor performed a punch biopsy which revealed ulcerated basal cell carcinoma (BCC). On examination, she had good pulses and there were no perineural symptoms nor signs nor regional lymph adenopathy. The patient elected to have radiotherapy as she had undergone multiple surgeries for skin cancers in the past few months and was suffering from surgical fatigue.
The patient was treated with the same quality beam as in Case 1 but to a total dose of 47.5 Gy in 19 fractions. She was originally prescribed 50 Gy in 20 fractions, but developed pain and a chyle leak towards the end of the treatment and so her treatment was stopped one fraction before the planned full prescription. The chyle leak and pain decreased over the next few weeks following radiotherapy (Figure 5A&5B), and she achieved a CR.
Figure 5 Lateral right ankle.
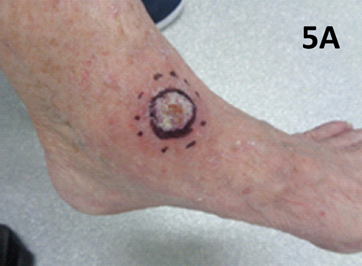
Figure 5A Case 5 at presentation showing a 2.5 cm lesion arising on the lateral right ankle which was biopsy proven to be BCC.
An important learning is that lower leg lesions particularly around the ankle can lead to chyle leaks which may take months to heal.
Case 6
A 97-year-old lady had a six-month history of a rapidly increasing but painless lesion on the right lateral calf. The patient’s past medical history was important – she had a pacemaker and was also anticoagulated. A biopsy of the lesion revealed cSCC. The plastic surgeon involved in the patient’s care requested treatment with radiotherapy. On presentation to the radiotherapy department, the 6.0 cm lesion was oozing but not bleeding and was mobile on deeper structures. There were no perineural symptoms or signs and no regional lymphadenopathy, which was confirmed on further imaging.
A multidisciplinary discussion was held between the physics and radiotherapy teams together with the radiation oncologist. Given the thickness of the lesion (1.1 cm), it was thought best to treat the patient with electron therapy rather than SXRT. She was planned using computer planning and treated with adaptive split course radiotherapy (ASCRT)10,11 to a phase one dose of 25 Gy in 10 fractions using nine mega electron volt (MeV) therapy. On reassessment eight weeks later, there had been a good partial response - the overall radial size of the lesion had not changed but its thickness had decreased to 0.53 cm with ASCRT.
A further multidisciplinary discussion was held to discuss whether the modality of treatment could be changed to SXRT for phase two of her treatment. It was thought wise to continue with electron therapy. To further decrease the lesion’s thickness, the second phase was initiated with six MeV radiotherapy eight weeks after the completion of phase one. The patient received a further 25 Gy in 10 fractions. The lesion thus received a total of 50 Gy in 20 fractions over a three-month period. Phase one used 1.0 cm of bolus, phase two used 5.0 mm (Figure 6).
Figure 6 Locally advanced cutaneous SCC of lower leg.

Figure 6A Right lateral calf lesion at first presentation for radiotherapy.
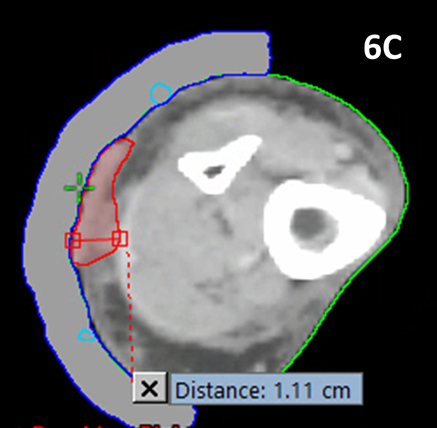
Figure 6C Planning scan for the first phase of MeV radiotherapy. The red contour is the gross tumour volume (GTV).6 The maximum tumour thickness at this time point was 1.1 cm.
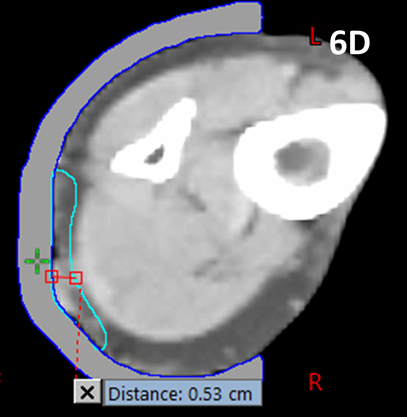
Figure 6D Planning scan for phase two of MeV treatment. The image is taken at a similar level as in Figure 6C. The GTV contour for phase two is shown in blue and the maximum thickness is 0.53 cm. The patient’s tumour had reduced by over half its thickness with ASCRT.
Case 7
A well 73-year-old lady presented with a 12-year history of a lesion on the anterior of her lower third right lower leg just over the tibia. It measured 3.5 cm by 4.0 cm in size. It was freely mobile over deeper structures. There were no perineural signs nor regional lymphadenopathy. It had been treated with imiquimod 5% six years prior with good effect but recurred. A repeat biopsy a year prior showed persistent superficial basal cell carcinoma. A repeat course of imiquimod was tried with initial success, but the lesion recurred yet again. Surgery was declined because of the presence of idiopathic bilateral lymphoedema which was well palliated by regular diuretic treatment. The patient was referred to radiotherapy for an opinion and consented to undergo treatment.
The patient was treated with the same beam as in Case 1. She was treated to a total dose of 60 Gy in 30 fractions. The applicator used measured 5.0 cm by 4.0 cm (Figure 7). Figure 7A shows the lesion on presentation for radiotherapy. Figure 7B shows the lesion at the completion of SXRT. Unfortunately, she developed cellulitis through the broken skin which produced an ulcer measuring 4.0 cm by 3.5 cm in maximum size. This needed several courses of oral and intravenous antibiotics as well as elevation and rest. Figure 7C is a photo taken prior to commencing antibiotics. Figure 7D is post antibiotics (four weeks later). Note that the lesion was beginning to resolve, and its diameter measured 3.0 cm by 3.5 cm compared to the previous photo. The lesion was also shallower at this time point. The patient continues to recover well at the time of writing. An important learning is that follow-up is needed, especially to ensure that any acute toxicities have resolved before discharge from the radiation oncology service.
Figure 7 Radio necrotic ulcer following SXRT to the lower leg.
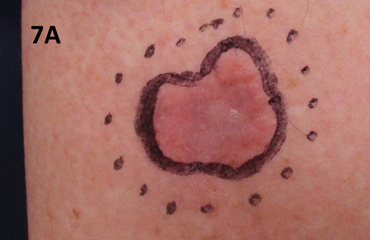
Figure 7A At presentation. A recurrent 3.5 cm by 4.0 cm lesion on the anterior of the lower leg over the tibia. The planned SXRT field is marked as a dotted line.
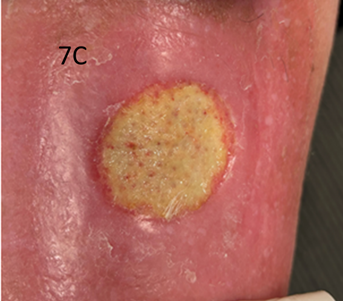
Figure 7C Image prior to the administration of antibiotics. Note the erythema out of field and the swollen nature of the leg. The ulcer measured 4.0 cm by 3.5 cm in diameter.
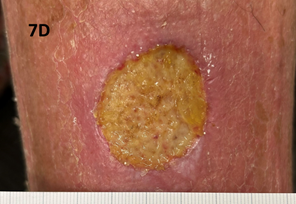
Figure 7D Image taken four weeks after the previous photo (Figure 7C). The lesion was beginning to resolve and measured 3.0 cm by 3.5 cm compared with Figure 7C.
Ears
Case 8
A fit 83-year-old male presented with a rapidly growing lesion on the superior border of the right pinna measuring 4.0 by 3.0 cm in size. The histopathology was positive for cutaneous squamous cell carcinoma. The patient was treated with SXRT to a total of 14.4 Gy in 6 fractions using an applicator measuring 6.0 cm by 5.0 cm. However, it was noted that the lesion continued to grow during treatment (Figure 8A-8D). In particular, the thickness of the lesion increased to such an extent that the superior pinna began to lean laterally under the influence of gravity (Figure 8E). It was thought too thick to continue with SXRT. The patient was therefore switched to a MeV technique to a dose of 50 Gy in 20 fractions. At the end of treatment, the legion was still not clinically fully under control and a further 10 Gy in 4 fractions was added using a MeV technique. In total, the patient received 74.4 Gy using different modalities but achieved a CR which continued at one year of follow-up (Figure 8F).
An important learning is the need to assess the response to treatment during the treatment course and to adapt as required.
Figure 8 Mixed modality treatment of cSCC of the pinna.
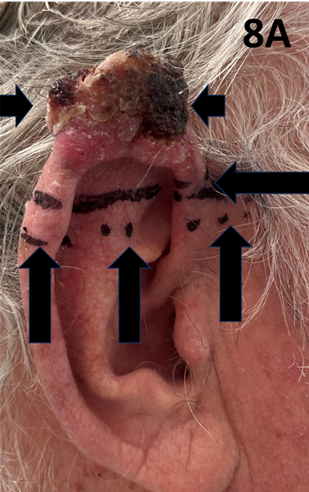
Figure 8A cSCC of the pinna as defined by short horizontal arrows. The lesion’s border is defined by the long horizontal arrow, and the SXRT field is marked by the dotted line above the vertical arrows.
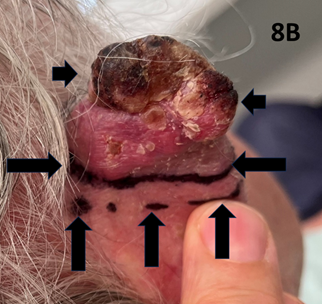
Figure 8B Photo showing the medial side of the pinna with cSCC of the pinna defined by the short horizontal arrows. The lesion’s border is defined by the long horizontal arrows, and the SXRT field is marked by the dotted line above the vertical arrows.

Figure 8C After receiving 14.4 Gy in six fractions using a 6.0 cm by 5.0 cm SXRT applicator the lesion was noted to be growing rather than regressing.
This case series set out to document some important experiences and learnings aimed at clinicians new to SXRT.
Shielding is one such learning. Appropriate shielding to protect healthy structures can greatly assist patients as demonstrated in Case 1 (lip). Typically, lead is used to collimate the primary field to shield unwanted dose to normal tissue, including scatter. Due to occupational health and safety concerns from lead poisoning, we use a non-toxic lead equivalent sheet called Attenuflex™ (3Done™, Queensland, Australia). The material is a thermoplastic sheet embedded with a tungsten carbide mixture. The thickness of the sheet is 1.6 mm, and it has a surface density of 15 kg/m2. The transmission is 0.5% for a 1.9 mm Al 100kV beam energy. Since the material is flexible and non-toxic, it is suitable to be placed directly onto the patient’s skin.
Our experience has also shown that the dose of radiotherapy can be titrated according to the acute effect of the radiotherapy on the cancer. For the lower legs, for example, chyle leaks can happen but resolve with time, and the use of adaptive split course radiotherapy may decrease the thickness of a lesion rather than show a decrease in radial dimensions.
Through these cases, we have also demonstrated that follow-up until any acute toxicities have resolved is important before discharging patients, and that regular on-treatment reviews should be performed as SXRT may not remain the most appropriate modality if the lesion changes during the course of therapy.
Finally, a real and practical advantage of SXRT lies in the versatility of the SXRT machine itself. Patients with mobility or pain issues do not need to be transferred from their bed or wheelchair to a treatment ‘couch’, making treatment relatively more comfortable and the workflow and set-up process more efficient (Figure 9).
SXRT provides an effective alternative to surgery in the definitive treatment of selected skin cancers and facilitates patient positioning due to the versatility of the machine. Clinicians embarking on an SXRT program should ensure adequate patient shielding and nutrition and understand that some tumours may be more radiation sensitive than others, as inferred from their macroscopic appearance. Regional lymph nodes should also be examined regularly to detect progression during treatment. It is hoped that our experience will help clinicians new to SXRT to deliver high quality care.
We are indebted to the patients and staff at Icon Cancer Centre Revesby for enabling us to document these experiences. We wish to thank Aileen Eiszele of A&L Medical Communications for writing and editing assistance.
We wish to gratefully acknowledge Xstrahl Ltd (UK) for funding the medical writer and open access publication.
The authors have no conflicts of interest to declare.

©2024 Fogarty, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.