International Journal of
eISSN: 2574-8084


Review Article Volume 9 Issue 5
1Department of Radiology, Jeevandeep Diagnostics, India
2Department of Radiology, Dr. D. Y. Patil Medical College, Hospital & Research Center, India
3Department of Radiology, LeBonheur Hospital, USA
Correspondence: Dr. Som Biswas, Department of Radiology, LeBonheur Hospital, UTHSC, Memphis, Tennesse, USA
Received: May 23, 2022 | Published: December 30, 2022
Citation: Awal SS, Prasad N, Biswas S. CT evaluation of aortic dissection and other acute aortic syndromes: An Update. Int J Radiol Radiat Ther. 2022;9(5):159-165. DOI: 10.15406/ijrrt.2022.09.00343
Acute aortic syndromes consist of aortic dissection, intramural hematoma, penetrating aortic ulcer, and ruptured aortic aneurysm. Acute aortic syndromes are considered a clinical emergency with patients presenting with severe chest pain. MDCT is considered as the investigation of choice to evaluate and classify acute aortic syndromes. Correct diagnosis and prompt management of acute aortic syndromes are imperative to reduce mortality and morbidity. Hence, imaging departments must have a basic understanding of their pathophysiology, imaging characteristics, and possible complications, this article reviews the updates in CT imaging of these syndromes and their pitfalls.
Keywords: acute aortic syndromes, aortic dissection, intramural hematoma, penetrating aortic ulcer, aortic aneurysm
AAS, acute aortic syndromes; IMH, intramural hematoma; PAU, penetrating atherosclerotic ulcer; MDCT, multidetector computed tomography; TEE, transesophageal echocardiography; MRI, magnetic resonance imaging; AD, aortic dissection; CTA, computed tomography angiography; MRA, magnetic resonance angiography; TAA, thoracic aortic aneurysm
Acute aortic syndromes (AAS) comprise of clinical emergency conditions that involve the aorta, namely: aortic dissection, intramural hematoma (IMH), penetrating atherosclerotic ulcer (PAU), and ruptured aortic aneurysm (Figure 1). Patients may present with severe chest pain, often described as ‘tearing’ in character.1,2 This has been classically described as ‘aortic pain’.1,3 Other symptoms include sweating, breathlessness, syncope, hypertension, or hypotension4 The incidence of the acute aortic syndrome is 2.6 to 3.5 cases per 100,000 person/year with a male preponderance.5,6 Timely diagnosis on imaging and prompt management are imperative as mortality in AAS increases by 1 - 2% per hour7. Multidetector computed tomography (MDCT) is the investigation of choice to diagnose AAS, having high sensitivity and specificity reaching up to 100%.4,8,9 The advent and use of gating techniques such as retrospective and prospective gating have made aortic imaging easier and far superior to ungated CT techniques. Transesophageal echocardiography (TEE) and Magnetic Resonance Imaging (MRI) are useful and complementary investigations in diagnosing AAS.4
The aorta is the largest human artery, originating from the left ventricle, it is divided into 5 anatomical segments– aortic root, ascending aorta, aortic arch, descending thoracic aorta, and abdominal aorta (Figure 2). Ascending aorta extends from the aortic root to the origin of the brachiocephalic trunk. The aortic root is the part that contains the aortic valve, annulus, and sinuses. The aortic arch extends from the brachiocephalic trunk to the ligamentum arteriosum. The descending aorta extends from the ligament arteriosum to the diaphragmatic aortic hiatus. The abdominal aorta extends from the aortic hiatus to its bifurcation into bilateral common iliac arteries. The aortic isthmus is situated just distal to the origin of the left subclavian artery.
The aortic wall is composed of three layers – intima, media, and adventitia (Figure 2). Tunica intima is the inner layer while adventitia is the outermost layer.10–12
Mechanisms weakening the media layer of the aortic wall can lead to the formation of an aneurysm. This, in turn, can further lead to aortic dissection, intramural hematoma, and/or aortic rupture/ulcer.5,6
Risk factors & clinical presentation
The most common risk factors for the development of AAS are listed in (Table 1).5,6 The clinical symptoms of AAS include severe chest pain, sweating, breathlessness, syncope, hypertension, or hypotension. The characteristic chest pain has been classically described in the literature as ‘aortic pain’. It is often sudden in onset and severe in nature.1,3,4
Atherosclerosis and dyslipidaemia |
Moderate to severe long-standing hypertension |
Connective tissue disease & vascular disorders |
Marfan syndrome, Ehlers-Danlos syndrome |
Hereditary thoracic aortic aneurysm or aortic dissection |
Turner syndrome |
Coarctation of aorta, Bicuspid aortic valve |
Inflammatory conditions |
Behcet’s disease |
Giant cell arteritis |
Takayasu arteritis |
Syphilitic aortitis |
Ormond disease |
Iatrogenic |
Aortic catheterization |
Aortic surgery |
Cardiac valvular surgery |
Traumatic aortic injury |
Chronic smoking |
Pregnancy |
Table 1 Risk factors for acute aortic syndromes
Aortic dissection
The most common type of AAS is aortic dissection (AD).13 Aortic dissection involving the thoracic aorta is the most common cause of the aortic emergency. It is characterized by the formation of an intimal tear which leads to blood entering the medial layer of the aorta wall (Figure 1). This causes longitudinal separation of the aortic lumen into a true aortic lumen and a false aortic lumen (Figure 4).8,9 The most common location for the initiation of this dissection is usually near the root of the ascending aorta.14
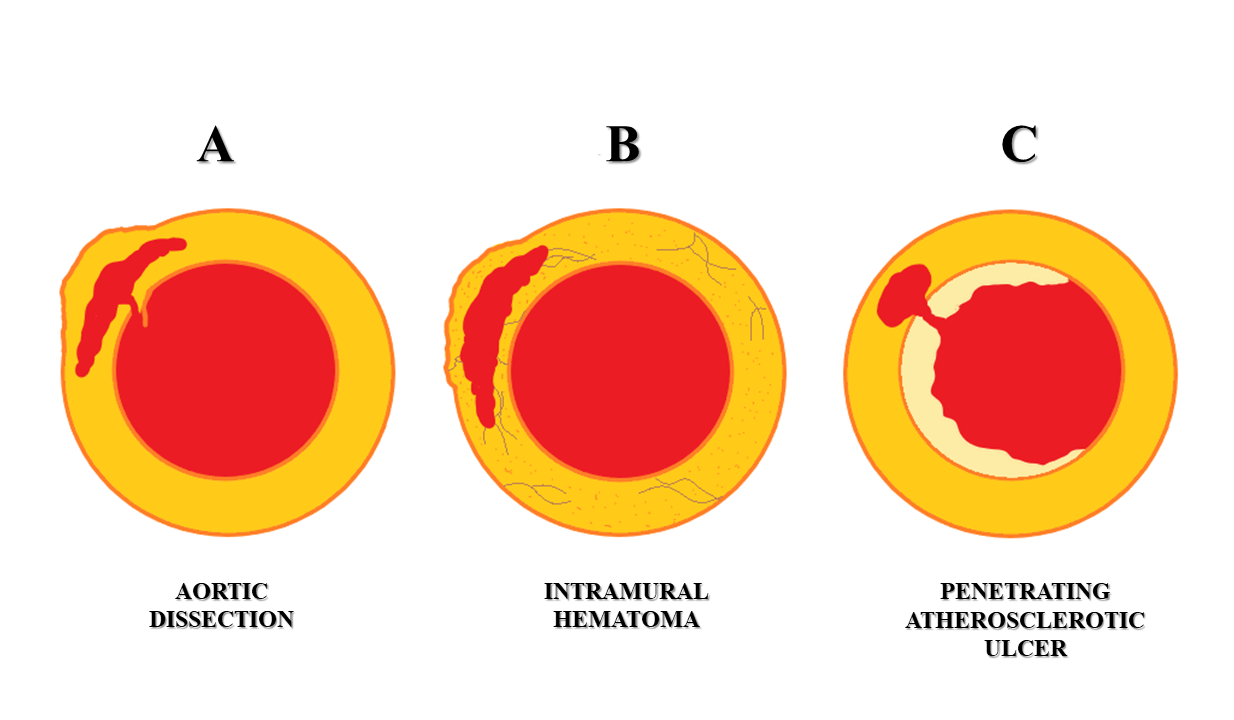
Figure 1 Schematic representation of acute aortic syndromes. A – Aortic dissection. B - Intramural hematoma. C - Penetrating atherosclerotic ulcer.
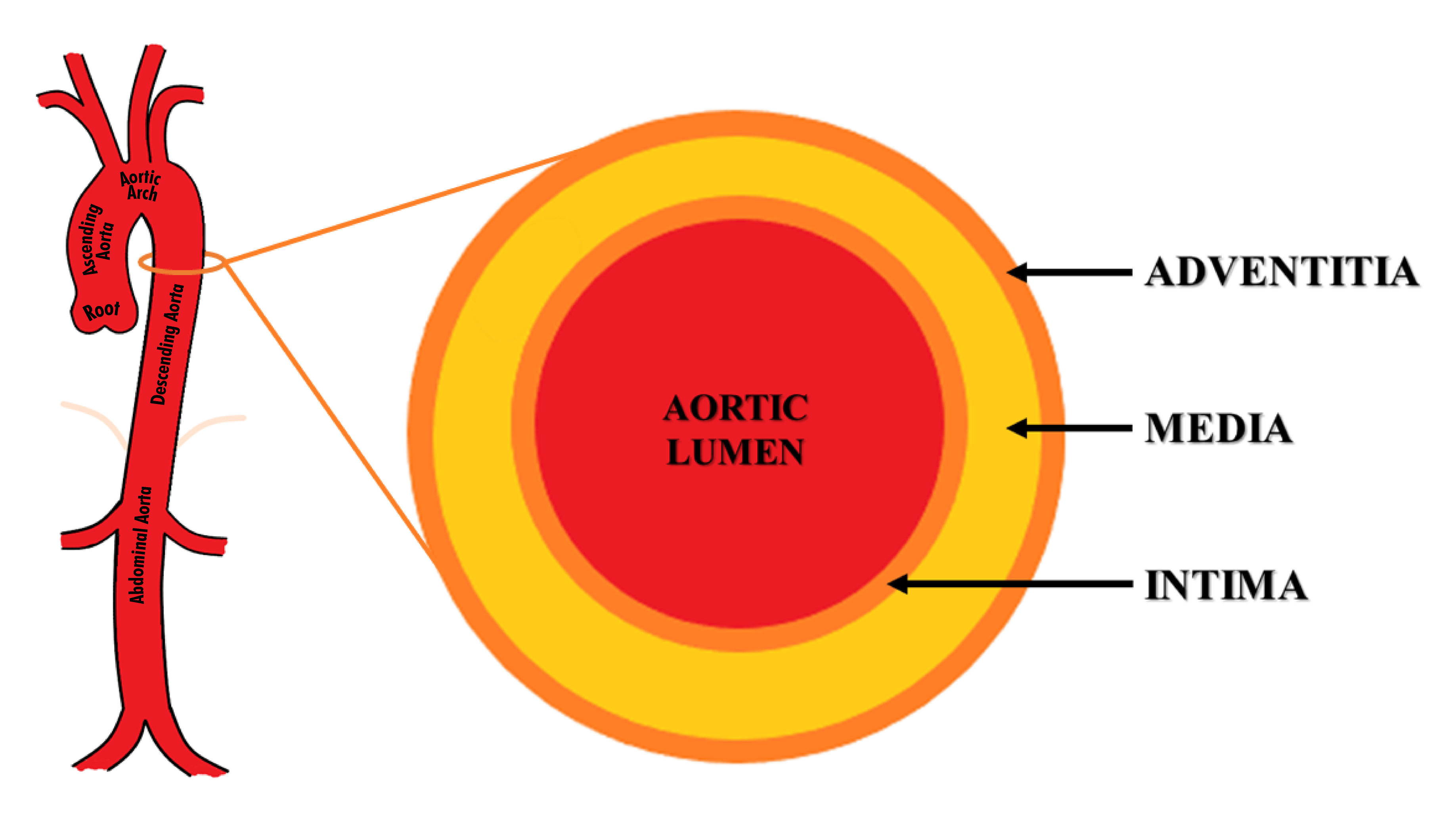
Figure 2 Structure and anatomy of the aorta. The aorta is divided into 5 anatomical segments– aortic root, ascending aorta, aortic arch, descending thoracic aorta, and abdominal aorta. Its wall is composed of three layers – intima, media, and adventitia.
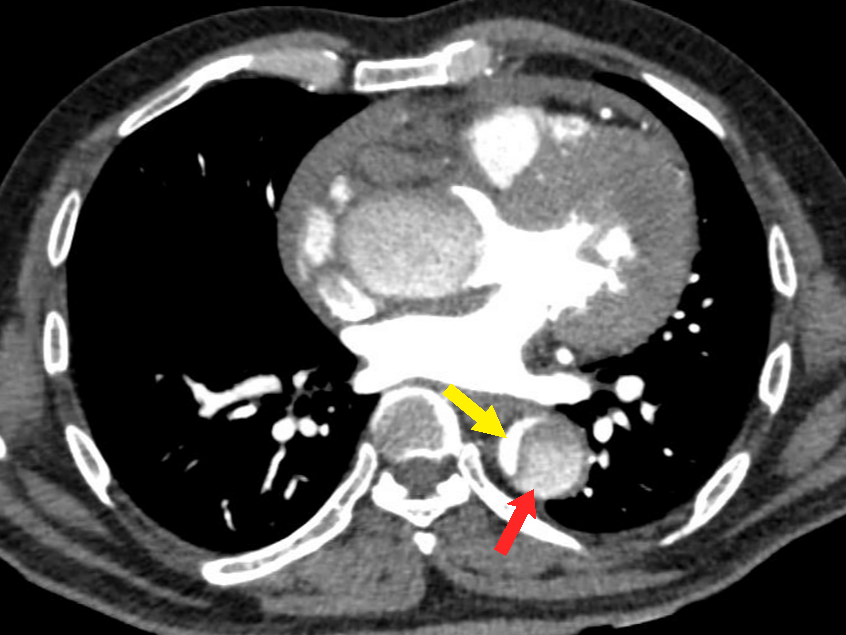
Figure 4 Axial CT angiography image in the arterial phase shows an aortic dissection of the descending aorta with a small-sized true lumen (yellow arrow) and a larger false lumen (red arrow). The true lumen shows more enhancement as compared to the false lumen.
Currently, two classification systems are used to describe and classify the types of aortic dissection according to its location and extent: DeBakey classification and Stanford classification (Figure 3). Both these classifications help segregate the cases that need surgical repair from the ones that can be managed medically.2,6,15,16
The DeBakey classification classifies aortic dissections into 3 types: DeBakey type I involves the ascending and descending aorta, DeBakey type II involves only the ascending aorta and DeBakey III involves only the descending aorta, sparing the aortic arch and ascending aorta.
The Stanford classification divides aortic dissections into 2 types: Stanford type A involves the aorta proximal to the origin of the left subclavian artery (i.e., ascending aorta or aortic arch), irrespective of descending aortic involvement (Figures 5a & 5b). It may involve the great vessels (Figure 6). Stanford type B involves the aorta distal to the origin of the left subclavian artery (Figure 7) (Figures 8a-8b). Stanford type B can be managed medically, but Stanford type A needs urgent surgical repair to avoid life-threatening complications. Stanford type A corresponds to DeBakey types I and II while Stanford type B corresponds to DeBakey type III.6,9,14 Stanford type A (DeBakey type I and II) aortic dissections are more common, accounting for two-thirds of cases. They require urgent surgical repair as they have a mortality rate of 1% to 2% per hour after the onset of symptoms. Stanford type B (DeBakey type III) aortic dissections are usually managed conservatively, in the absence of any complications.2,5,6,9,17
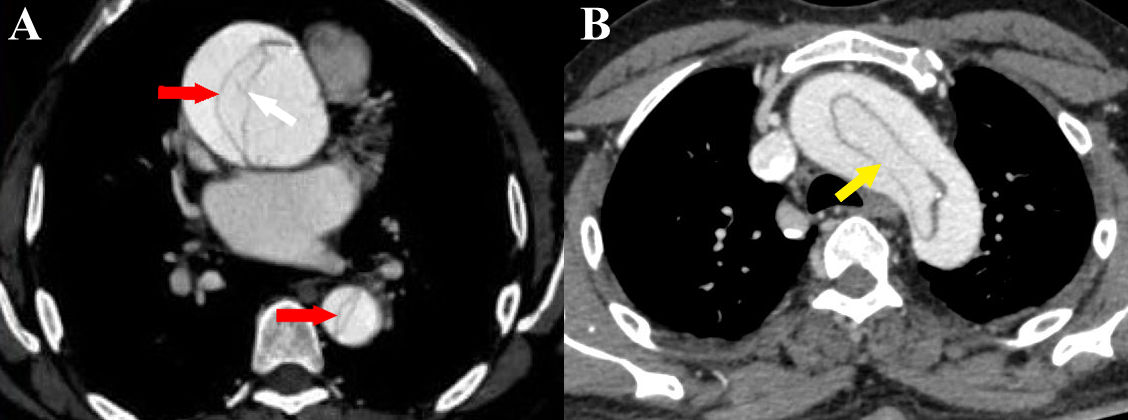
Figure 5 a-b Stanford type A aortic dissection. (a) Axial CT angiography image shows the dissection flap (red arrows) in ascending and descending aorta. Also seen are linear hypodense structures within the false lumen (white arrow) with similar attenuation as the intimal flap (“Cobweb sign”) [courtesy of Dr Suhas C. M, final year resident, MD Radiodiagnosis, Ms Ramaiah Medical College, Bengaluru, India]. (b) Axial CT angiography image shows a circumferential flap with oval morphology (“windsock” sign) involving the aortic arch (yellow arrow).
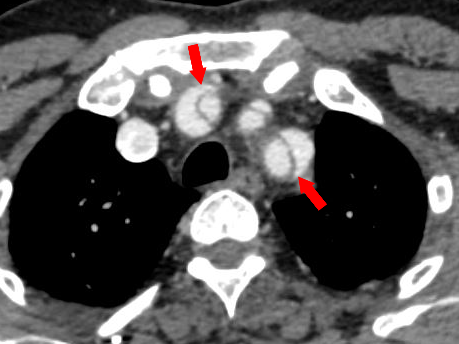
Figure 6 Axial CT angiography image of Stanford type A aortic dissection shows dissection flaps involving the great vessels. Note the “beak sign” (red arrows).
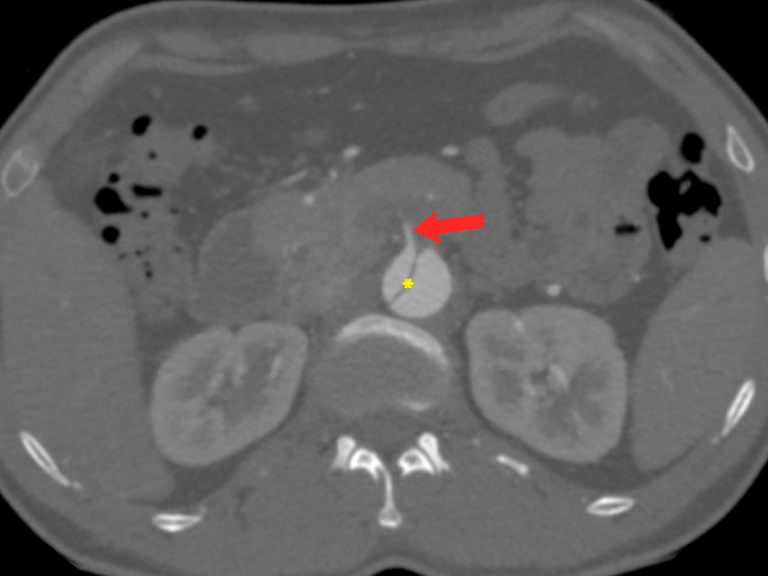
Figure 7 Stanford type B aortic dissection. Axial CT angiography image demonstrates an abdominal aortic dissection with extension into the superior mesenteric artery (red arrow) and a thrombus just beyond its origin. It is vital to check the origin of the main aortic branches as it can have serious prognostic implications. Note the intimal flap (yellow asterisk) separating the true lumen and false lumen.
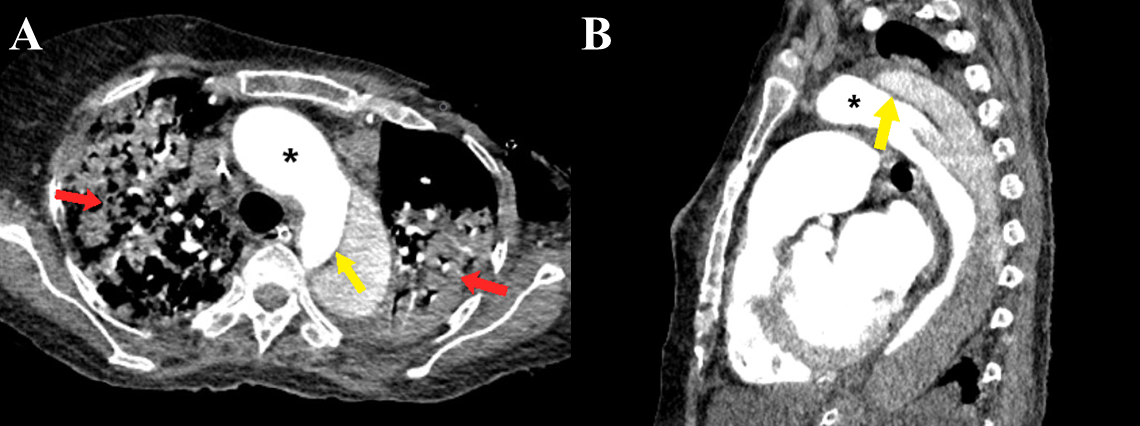
Figure 8 a-b Stanford type B aortic dissection. Axial (a) and sagittal (b) CT angiography images show the dissection flap (yellow arrows) involving the descending thoracic aorta distal to the origin of the left subclavian artery. Large consolidations are also evident in bilateral lungs (red arrows). Note the greater enhancement within the true lumen as compared to the false lumen (black asterisk).
Imaging findings
Imaging plays a key role in early diagnosis and delineating the extent of aortic dissection. Imaging should aim to establish the diagnosis, delineate the extent of the dissection, and describe the patency of the false lumen. An initial plain radiograph may be normal or show non-specific findings like mediastinal widening, medial displacement of atherosclerotic aortic calcification, or changes in aortic contour.8,18
MDCT is considered superior to MRI and TEE in the evaluation of AD.4,6,19,20 Non-contrast CT may demonstrate mural hematoma or medial displacement of intimal aortic calcification.9 It also has the advantage of having a shorter acquisition time and better availability as compared to MRI.17
Contrast-enhanced CT angiography (CTA) findings of AD include the presence of an intimal flap separating the true lumen from the false lumen, present in approximately 70% of AD cases (Figure 5a). Identification of true lumen is essential to the diagnosis of AD, for proper planning of endovascular intervention (Table 2). Features in support of true lumen are a smaller size than false lumen, calcifications along its outer wall or intimal flap, contiguity with unaffected aortic lumen, more enhancement as compared to the false lumen (Figure 4) (Figure 8a, 8b). Features in support of false lumen are larger lumen size than true lumen, non-communication with the unaffected aorta, less enhancement as compared to the true lumen, “cobweb sign” and “beak sign”.6,17,21 “Cobweb sign” was described by Williams et al as “residual ribbons of media that have incompletely sheared from the aortic wall during the dissection process” 22 They are seen as thin, linear filling defects of low attenuation within the false lumen (Figure 5a). “Beak sign” was described by Le Page et al as “the cross-sectional imaging manifestation of the wedge of hematoma that cleaves a space for the propagating false lumen” (Figure 6) (Figure 9).23 Occasionally, a circumferential flap may form because of dissection involving the entire aortic intima & subsequent intimointimal intussusception. The true lumen takes a fusiform or a cylindrical shape and gives a “windsock appearance” (Figure 5b).17
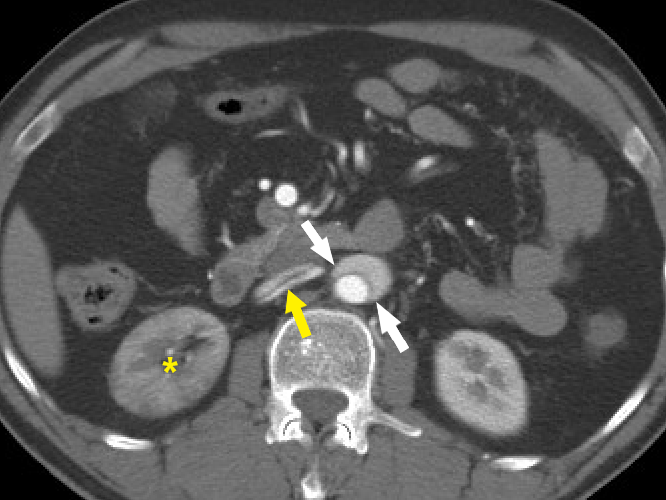
Figure 9 Beak sign of aortic dissection. Axial CT angiography image demonstrates the “beak sign” (white arrows) as an acute beak-like angulation formed by the false lumen. The dissection flap is seen extending into the right renal artery (yellow arrow). The right kidney is seen to enhance lesser (yellow asterisk) compared to the left kidney.
TRUE LUMEN |
FALSE LUMEN |
Smaller in size than the false lumen |
Larger in size than the true lumen |
Contiguous with unaffected aortic lumen |
Non-communication with the unaffected aorta |
More enhancement compared to false lumen |
Less enhancement compared to the true lumen |
Calcifications along its outer wall or intimal flap |
|
|
“Cobweb sign” |
|
“Beak sign” |
Table 2 Differentiating features of the true lumen and false lumen
An ideal CT report of AD should mention the parameters listed in Table 3. TEE is considered highly specific and sensitive for the identification of an Intimal flap, but its value remains limited due to its poor availability, lack of expertise, and invasive nature.17
|
|
|
|
|
|
|
|
Table 3 Ideal CT report of aortic dissection
Contrast-enhanced magnetic resonance angiography (MRA) is usually reserved for patients with chronic aortic dissections and stable patients. It is advantageous over CTA in providing a dynamic evaluation of the dissection, good spatial resolution, lack of ionizing radiation, and multiplanar capabilities. However, it has limited capability in medically unstable patients owing to its long acquisition time, in patients with electronic devices, and follow-up post endovascular intervention.17
Intramural hematoma
Aortic intramural hematoma (IMH) refers to a contained acute hematoma within the medial layer of the aortic wall (Figure 1). It is thought to occur due to a spontaneous rupture of the vasa vasorum of the aortic media.6,9,24 It is considered by some as an atypical form of aortic dissection or even a precursor of aortic dissection.4,24 It accounts for approximately 5-15% of all cases of AAS.6 The clinical manifestations of IMH are often indistinguishable from an aortic dissection. However, the imaging findings of IMH are distinct from aortic dissection.1,24 IMH has a variable prognosis with 10% of cases showing spontaneous regression while up to 47% of cases may progress to aortic aneurysm. IMH is also classified according to the Stanford or DeBakey classification. Stanford type A IMH may require surgery while in Stanford type B is usually managed medically.6,9
Imaging findings
CT is an excellent imaging modality for the diagnosis of IMH with sensitivity and negative predictive value approaching 100%. Non-contrast CT is extremely useful in the diagnosis of IMH [9]. On non-contrast CT, IMH is seen as a crescentic hyperdense (60-70 HU) thickening within the aortic wall, usually more than 7mm in diameter but generally less than 15mm (Figure 10a).4,8,9,24,25 Intimal calcifications, if present, can be seen displaced inwards, differentiating it from a mural thrombus.9,24,25 The majority of IMH involve the descending aorta.4
On contrast-enhanced CT, the crescentic thickening remains unenhanced (Figure 10b). IMH is characterized by the absence of intimal disruption which is the characteristic feature of AAD. Parameters to be included in an ideal report of IMH have been summarized in (Table 4).
|
|
|
|
Table 4 Ideal CT report of intramural hematoma
TEE has high sensitivity and specificity in the detection of IMH. However, it is operator-dependent, invasive, and lacks complete visualization of the thoracic aorta.24
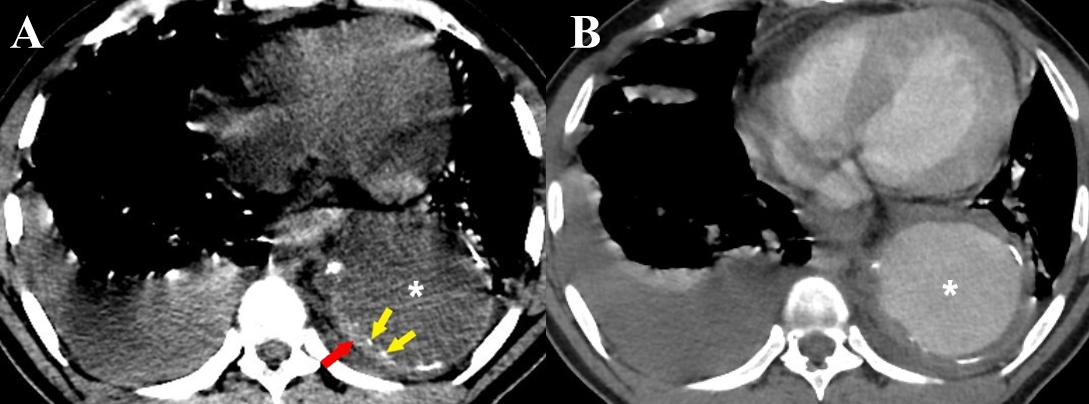
Figure 10 a-b Intramural hematoma. (a) Axial non-contrast CT thorax image in a case of thoracic aortic aneurysm (white asterisk) shows a crescentic thickening of high attenuation within the wall of the descending thoracic aorta (red arrow). Intimal calcifications are seen displaced inwards (yellow arrows). (b) A contrast-enhanced CT thorax image of the same patient shows no enhancement of the IMH. Bilateral pleural effusions are also seen in both images (a) & (b).
MRA is usually reserved for patients with chronic aortic dissections and stable patients. It is advantageous over CTA in providing a dynamic evaluation of the dissection, good spatial resolution, lack of ionizing radiation, and multiplanar capabilities. However, it has limited capability in medically unstable patients owing to its long acquisition time, in patients with electronic devices, and follow-up post endovascular intervention.17
MRI reaches a sensitivity of 100% in the diagnosis of IMH. Its advantage remains in follow-up studies due to its lack of ionizing radiation and functional imaging of the aortic valve. However, its application in IMH is limited by a longer acquisition time and poor availability.25
Penetrating atherosclerotic ulcer
Penetrating atherosclerotic ulcer (PAU) refers to an ulcerating atheromatous plaque that penetrates through the internal elastic lamina of the aortic wall into its medial layer (Figure 1). It contributes approximately 2-8% of all cases of AAS.6,26-28 It is typically seen in the elderly population with etiological factors such as hypertension, atherosclerotic disease, peripheral arterial disease, abdominal aortic aneurysm, tobacco usage, and coronary artery disease.8,26,28,29 Paus is known to have an unfavorable prognosis and may lead to an aortic rupture leading to the need for urgent invasive management.6,27
The underlying pathology in PAU is a diseased intimal layer and not the media, as in the case of aortic dissection.6,30 During the early stages, an atheromatous lesion may ulcerate into the intimal layer. Eventually, the atherosclerotic ulcer may deepen and penetrate through the intimal layer into the media, hence the term “penetrating atherosclerotic ulcer”.28,29 PAU is most frequently seen within the descending thoracic aorta as an atherosclerotic aortic disease most commonly involves this segment.6,8,28,31
Imaging findings
Contrast-enhanced CT is the imaging modality of choice for diagnosing PAU in acute cases.6 On contrast-enhanced CT or MRI, the typical finding of PAU is a focal crater-like protrusion of the aortic wall opacified with contrast (Figure 11), often with jagged edges.6,8,26,28 There is the absence of an intimal flap or a false lumen. Associated atheromatous aortic plaques and calcifications are usually present.26,28,32 A high-density hematoma is often seen surrounding the PAU on non-contrast CT. Key points to be included in an ideal report of PAU have been summarized in (Table 5). A PAU with a neck measuring greater than 10mm or a diameter greater than 20mm is at a higher rate of progression and needs prompt intervention.8,31,33,34

Figure 11 Penetrating aortic ulcer. Axial CT angiography image shows a focal contrast-filled outpouching (yellow arrow) of the posterior wall of the suprarenal abdominal aorta.
|
|
|
|
Table 5 Ideal CT report of penetrating aortic ulcer
TEE is highly sensitive and specific in differentiating PAU from other AAS. It demonstrates a crater-shaped ulcer with jagged edges.32,35
Ruptured aortic aneurysm
An aortic aneurysm is defined as the focal pathological dilatation of the aortic wall, exceeding 1.5 times the normal expected diameter at the same segment. It may be focal or diffuse and may affect the thoracic aorta or the abdominal aorta.20
In the case of thoracic aortic aneurysms, dilatation of less than 50% over normal would be termed as aortic ectasia, whereas thoracic aortic aneurysm (TAA) is diagnosed when there is a minimum 50% enlargement of the aortic lumen, or when the aortic diameter is more than two standard deviations above the mean for the patient’s sex and age. Surgical intervention needs consideration for aneurysms more than 5 cm in diameter, and TAAs that increase in size >0.5 cm per year.20,36 An abdominal aortic aneurysm is defined as the dilatation of the abdominal aorta that is 50% more than the proximal normal segment or when it is more than 3 cm in maximum diameter.20,37
Sudden onset of pain in a patient with a known aortic aneurysm may indicate hemorrhage, dissection, or impending rupture. Prompt diagnosis of a ruptured aortic aneurysm or impending rupture is vital as it has a high mortality rate.38,39
CTA is the gold standard for imaging of aortic aneurysms and is very useful in depicting the extent of the aneurysm, involvement of arterial branches, pre-operative planning as well as signs of instability and rupture. MRA in comparison is costly, less widely available, has a longer imaging time, and has a less spatial resolution. Digital subtraction angiography, though required for management, does not always show true aneurysm size if there is a mural thrombus.
A large baseline aneurysm size, exceeding 7cm (Figure 12) and a rapid size progression of more than 10mm increase per year are associated with a higher risk for rupture.38,40,41 Additional CT findings that are predictors of aortic aneurysm instability include low thrombus-to-lumen ratio, luminal expansion with lysis of thrombus, “hyperattenuating crescent sign”, fissuration of thrombus, periaortic hemorrhage, a penetrating atherosclerotic ulcer, focal discontinuity of intimal calcification (Figure 13), and the “draped aorta sign”.38,41 Correct documentation of any pleural effusion, pericardial effusion, or periaortic hematoma is a must as they are associated with increased risk of progression and mortality.42 Primary signs of aortic aneurysm rupture include retroperitoneal hematoma, periaortic fat stranding, and active extravasation of contrast material at the site of rupture.38,43
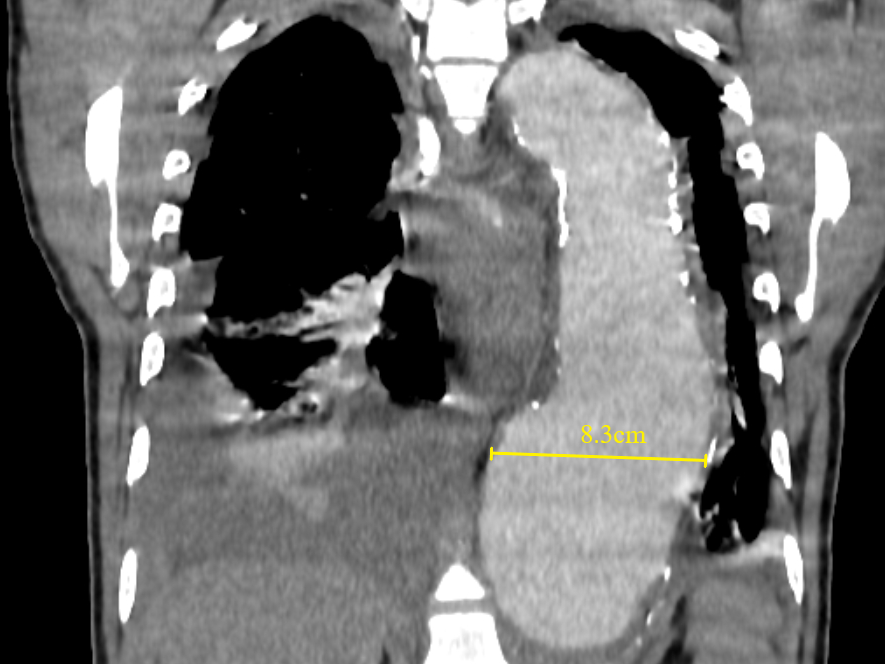
Figure 12 Coronal contrast-enhanced CT thorax image shows a large thoracic aortic aneurysm (measuring approximately 8.3cm in maximal diameter). Baseline aneurysmal diameter exceeding 7cm is at a higher risk of aortic rupture than smaller aneurysms. Pleural effusions, as seen in this case, must be reported as they are associated with instability and progression.
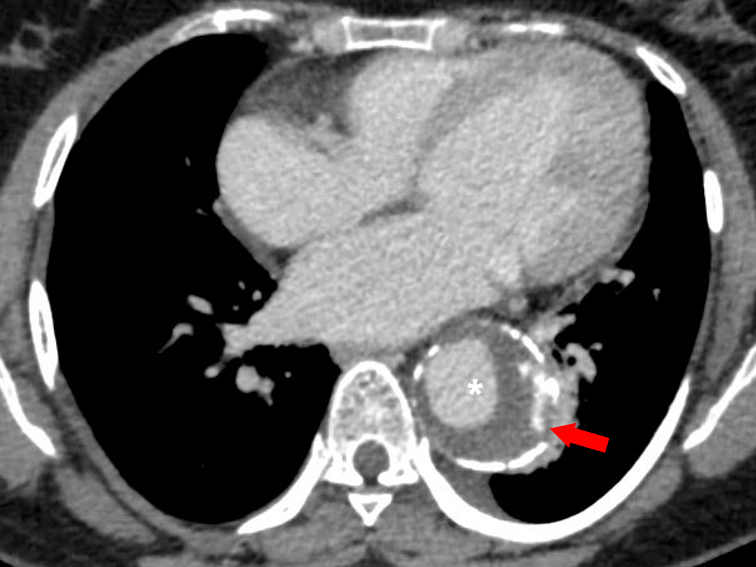
Figure 13 Axial CT angiography image in the delayed phase shows an aortic aneurysm (white asterisk) in the descending aorta with displaced intimal calcifications and a break in the rim of calcifications in its lateral aspect (red arrow). Focal discontinuity of intimal calcification is a predictor of aortic aneurysm instability.
“Hyperattenuating crescent sign” represents an acute hematoma within the aortic wall or mural thrombus and is a sign of impending rupture. It occurs because of dissected blood insinuating into the mural thrombus and eventually penetrating the aortic wall. It is seen as a peripheral crescent of high attenuation within the aortic wall or mural thrombus.38,43,44 The “draped aorta sign” is a vital sign of contained rupture of an aortic aneurysm. The posterior aortic wall is not identifiable as a distinct line rather appears draped or molded to the anterior aspect of the adjacent vertebral body.38,45
Recent advances include the development of software to measure abdominal aortic aneurysm diameter, volume, and enlargement. Few have proven volumetric analysis to be more useful than diameter measurement in the follow-up.46
Acute aortic syndromes encompass a set of aortic pathologies that have similar clinical presentation and etiology. Imaging modalities, especially CT, have proven indispensable in their diagnosis and further management. A basic understanding of the pathophysiology, and imaging characteristics are essential in prompt diagnosis and appropriate management of patients with acute aortic syndromes. There have been improvements in imaging of these syndromes, with improvement in CT machines. We provide updated images in this article.
None.
All authors contributed equally and there is no conflict of interest.
None.

©2022 Awal, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.