International Journal of
eISSN: 2574-8084


Case Report Volume 9 Issue 1
Department of Radiology, St John’s Medical College Hospital, India
Correspondence: Karthik Shyam, Department of Radiology, St. John’s Medical College Hospital, Bangalore, India – 560034, Tel 9448129802
Received: December 07, 2021 | Published: February 18, 2022
Citation: Shyam K. Case report: 50-year-old male with invasive aspergillosis of the native knee joint. Int J Radiol Radiat Ther. 2022;9(1):25-27. DOI: 10.15406/ijrrt.2022.09.00319
Septic arthritis is a disabling arthritis that destroys cartilage and joints due to infective inflammation. Early and prompt diagnosis is required to ensure an optimal outcome. Septic arthritis secondary to fungal aetiology is rare; commonly seen in immunocompromised and post-arthroplasty patients. We present a rare case of spontaneous fungal septic arthritis in a 50-year-old male, with imaging and laboratory features of the same.
Keywords: Arthritis, invasive aspergillosis, Knee joint
Septic arthritis is a common disabling arthritis that destroys cartilage and joints due to enzymes released by neutrophils, synovial cells, and bacteria.1 They need early and prompt diagnosis to ensure an optimal outcome, it is traditionally a clinical diagnosis based on local examination and athrocentisis.2 Septic arthritis is divided into two categories namely pyogenic and non–pyogenic arthritis, pyogenic arthritis is most commonly caused by Staphylococcus aureus, Neisseria gonorrhoeae, Klebsiella Pneumoniae, Candida albicans, and Serratia marcescens.3,4 Non–pyogenic arthritis is caused by tuberculosis, fungi like actinomyces, cryptococcus, coccidioides, Histoplasma and sporothrix and viruses like Haemophilus influenza and spirochetes.5–7 The causative organism can enter the joint space by invading the synovial membrane following a penetrating wound or after surgery like joint replacement, they can also enter the joint secondary to infection of adjacent soft tissues or via blood–borne infections. A focus of osteomyelitis in the adjacent bone can also lead to infectious arthritis.6–9
Septic arthritis secondary to fungal infection are rare, they are commonly seen in patients suffering from chronic disease, immunocompromised patients and post arthroplasty patients.10 Fungal infection can have a wide range of presentations from indolent to rapid destruction, in the case of indolent infection diagnosis can be very difficult due to the lack of significant host response.11 There may be a normal or minimal elevation of systemic inflammatory markers in case of fungal osteomyelitis, hence the history and physical examination are very crucial in diagnosis.12 Aspergillus is a ubiquitous fungal organism that can cause focal lung infection.13 Invasive aspergillosis is a life–threatening and rare opportunistic infection that is common in immunocompromised patient,14 the most common musculoskeletal manifestation of it is osteomyelitis. In children, the infection can occur due to contiguous spread from skin, lungs or sinus infection, in adults, the infection occurs due to hematogenous spread.15 The most frequent bone to be involved in the case of hematogenous spread is the lumbar vertebrae.16
Aspergillus fumigatus is the most common causative organism accounting for the majority of infections followed by aspergillus flavus.17 Septic arthritis caused by aspergillus in a non–immunocompromised and native joint has not been commonly reported in the literature. Aspergillus, though being a rare cause of septic arthritis, is associated with high morbidity and mortality18. We present a case of septic arthritis of the knee caused due to invasive aspergillus infection.
A 50–year–old male presented to the orthopaedic OPD with a history of left knee pain and swelling for 3 months, insidious in onset with no antecedent history of trauma or therapeutic intervention. There was a gradual progression of pain and swelling with a history of on and off fever, presently the patient was unable to bear weight and do daily activity. On local examination, the left knee appeared swollen with fullness in the supra and parapatellar region with mild local raise in temperature. There was a decreased range of motion secondary to pain.
Routine lab investigations were done, the patient had a normal total count (6.1 x 10 3 cells per cu. mm) decreased haemoglobin (8.2 g/dl) and decreased serum calcium levels. On further investigation, there were features of iron deficiency anaemia and vitamin D deficiency. The patient had normal renal and liver parameters with negative status for HIV and Hbs ag.
Radiograph of left knee acquired in AP and lateral views showed features of a severe narrowing of medial aspect, irregularity of the articular surface, osteophytes from articular surfaces and fullness in the suprapatellar recess region with soft tissue oedema – Likely due to effusion. There is no evidence of subchondral sclerosis. (Figure 1–2)
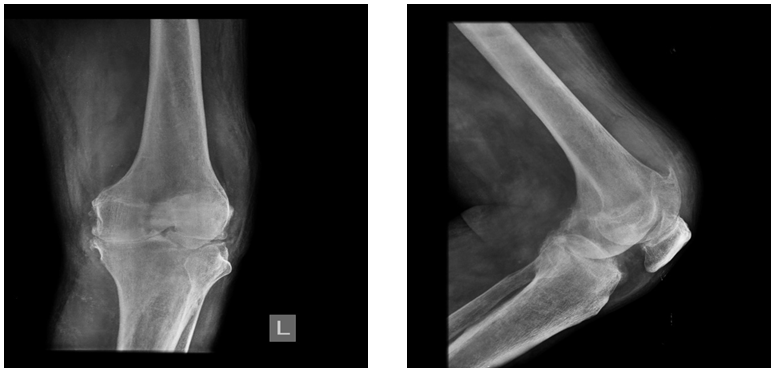
Figure 1&2 AP and Lateral radiographs of the left knee demonstrates imaging features of grade IV arthritis (modified Outer bridge classification). There is a severe narrowing of joint space (<3mm), with medial compartmental predominance. Also noted is subchondral and marginal osteophytosis in patella, bilateral femoral and tibial condyles with mild varus deformity. This could indicate the presence of a cartilage defect. Fullness in the suprapatellar recess region with soft tissue oedema is also seen.
The patient underwent a non–contrast CT of the left knee joint, there were features of bony erosion in the articular surface of femur and tibia, joint effusion, unequal joint space narrowing, and degenerative changes. (Figure 3–8)
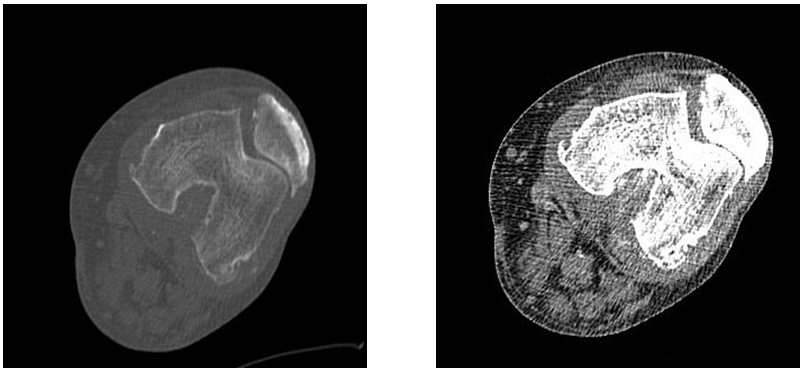
Figure 3&4 Axial sections of left knee joint in bone window and soft tissue windows demonstrating irregular patellofemoral joint space, multiple osteophytes from articular surfaces of femur and patella and joint effusion.
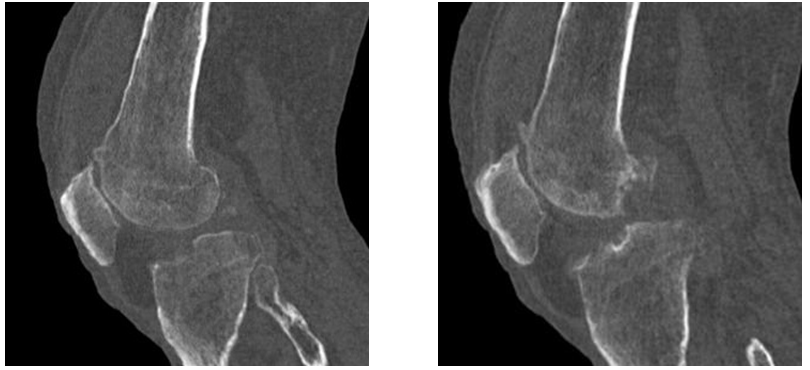
Figure 5&6 Sagittal sections of left knee joint in bone window demonstrating multiple foci of bony erosions in the lateral condyle of femur and tibia with multiple osteophytes from articular surfaces.
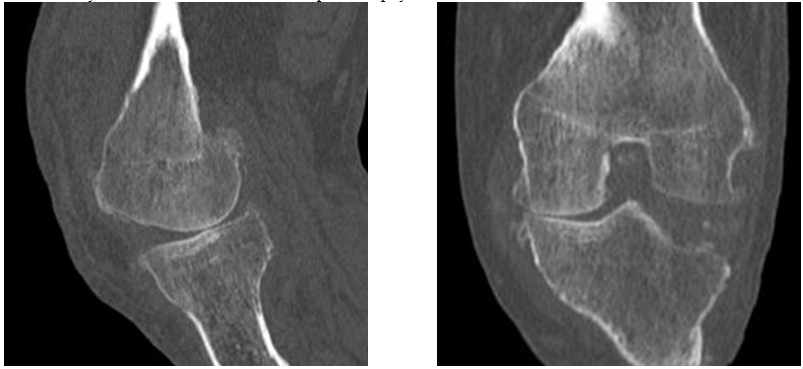
Figure 7&8 Sagittal and coronal section of the knee joint demonstrating narrowing of joint space adjacent to the medial femoral condyle and relative joint space widening in the lateral compartment with bony erosion of lateral tibial condyle.
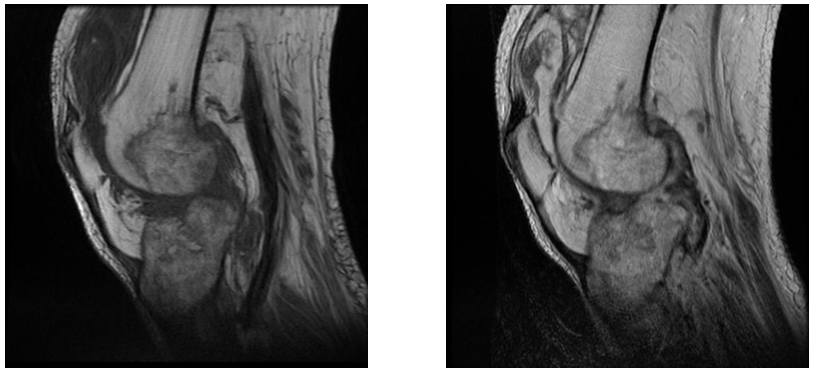
Figure 9&10 Sagittal T1 and T2 images of left knee demonstrates patchy marrow oedema in the articular surface, moderate joint effusion and synovial thickening.
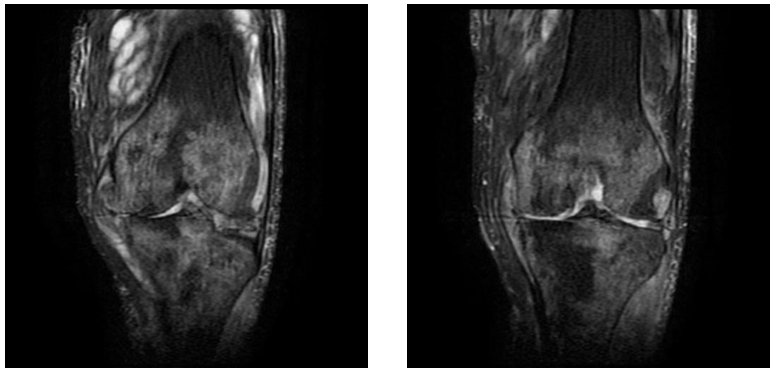
Figure 11&12 Coronal STIR images demonstrate patchy marrow oedema in the subchondral bone in both tibia and femur, irregular joint space narrowing, complex tear of the lateral menisci and irregularity of lateral tibial condyle.
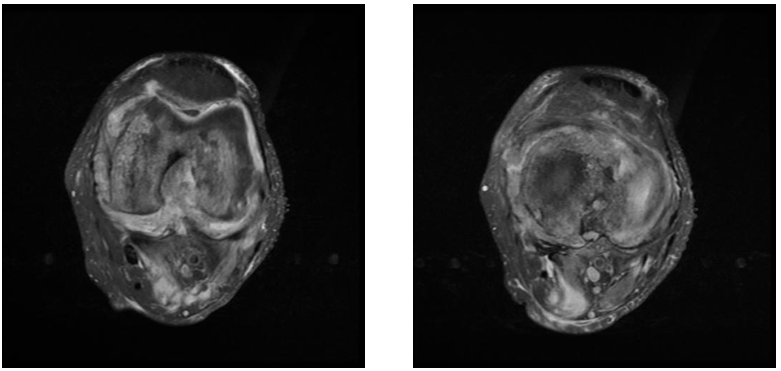
Figure 13&14 Axial PD images demonstrate significant synovial thickening, moderate joint effusion, and peri-articular inflammatory changes. A small collection was noted between the medial head of gastrocnemius and soleus muscles.
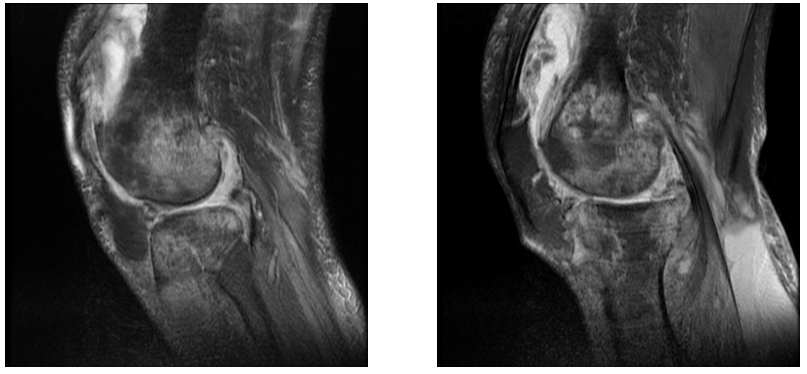
Figure 15&16 Sagittal PD images demonstrate features of cartilage erosion in the lateral femoral condyle, joint space reduction and marrow oedema in the articular surface.
The patient underwent non–contrast MR of the knee joint, there were complex tears of lateral and medial meniscus, significant synovial thickening, moderate joint effusion, features of bony and cartilage erosion, peri–articular inflammatory changes and patchy marrow oedema adjacent to the articular surface of tibia and femur.
The case presented two diagnostic dilemmas, the first was differentiating septic arthritis from inflammatory arthritis and the second was differentiating tubercular septic arthritis from pyogenic septic arthritis. There are overlapping features between septic and inflammatory arthritis, Features like bone erosions, bone oedema, and cartilage destruction, are seen more commonly in septic arthritis. Soft tissue oedema and enhancement are more common in the case of inflammatory arthritis19. Similarly, a study done by Choi et al tried to differentiate tuberculous (TB) arthritis from Rheumatoid arthritis (RA), the study demonstrated that in RA there can be uneven and significant synovial thickening or even mass formation and smaller sized erosions. In TB arthritis there can be larger bone erosion, rim enhancement of erosions and extra–articular cystic mass formation, however, the study did not find a significant difference between bone oedema caused due to TB and RA.20
Similarly differentiating TB from pyogenic arthritis is very difficult, in the case of TB arthritis, there is an increased prevalence of bone erosion, extra–articular extension of infection and lesser prevalence of marrow edema.21 In the case of pyogenic septic arthritis, there was a higher prevalence of marrow oedema changes,22 the lesser prevalence of bone erosion and extra–articular extension. The extra–articular extension leads to the formation of an abscess, on post–contrast images the abscess will show well defined and smooth margins in case of TB arthritis and irregular margins in case of pyogenic infection.23 Contrast–enhanced MR is very critical in differentiating marrow oedema from osteomyelitis, there will be enhancement of the involved area in case of osteomyelitis.24
Based on the imaging findings on bone/cartilage erosion, significant marrow oedema, irregular joint space narrowing, thickened synovium and effusion we had given the differential diagnosis of 1. Septic arthritis, 2. Tuberculous arthritis with degenerative changes and 3. Inflammatory arthritis but less likely possibility, the patient was advised contrast–enhanced MR. The patient underwent a synovial biopsy which showed features of angioinvasive aspergillosis. A retrospective examination of the images was done after biopsy findings, however, there were no helpful imaging features to ascertain the fungal cause of septic arthritis. Despite the findings discussed above, the features of inflammatory and septic arthritis overlap to a large extent and necessitate biopsy for confirmatory diagnosis. Contrast–enhanced MR is an excellent tool to guide clinicians and prevent undue delays in the diagnosis of cases, a delay in diagnosis can have a detrimental effect on the outcome.
None.
Authors declare no conflict of interest.

©2022 Shyam. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.