International Journal of
eISSN: 2574-8084


Research Article Volume 9 Issue 3
National Coalition of Independent Scholars, Battleboro, VT, USA
Correspondence: Marco Ruggiero, MD, PhD, National Coalition of Independent Scholars, 125 Putney Rd Battleboro, VT 05301, USA
Received: July 27, 2022 | Published: August 22, 2022
Citation: Ruggiero M. Application of ultrasonography to Neuro-COVID-19. Int J Radiol Radiat Ther. 2022;9(3):99-103. DOI: 10.15406/ijrrt.2022.09.00331
Aim: The aim of this study is to evaluate the role of ultrasonography in diagnosis and treatment of COVID-19, the disease caused by severe acute respiratory syndrome-related coronavirus 2 (SARS-CoV-2), with particular reference to the symptoms that are frequently observed in Neuro-COVID-19, a term that indicates the plethora of short- and long-term neurologic and psychiatric manifestations caused by, or associated with the disease. In a significant percentage of cases, neuro-psychiatric symptoms persist after recovery and long-term sequelae have been reported. SARS-CoV-2 can infect the brain through different routes and the damage can be direct, that is due to the virus itself, or indirect, that is associated with abnormal immune responses, inflammation, and hypoxia.
Methods: In this study, the brain was studied by transcranial ultrasonography. Analysis of brain specimens obtained from autopsy demonstrated the presence of the virus in a minority of cases and this leads to hypothesize that SARS-CoV-2 may hide in sanctuary sites in the central nervous system in analogy with what observed for HIV. The existence of sanctuary sites for SARS-CoV-2 has the potential to decrease the efficacy of antiviral therapies or vaccination and may even prevent complete eradication of SARS-CoV-2 from the infected organism.
Results: Transcranial ultrasonography demonstrated significant movements of the brain associated with the respiratory cycle. In 2017, a diagnostic and therapeutic procedure was proposed with the goal of identifying and treating pathogens hiding in sanctuaries that elude diagnosis and therapy. This procedure is based on clinical evaluation, diagnostic ultrasonography, therapeutic ultrasounds, and laboratory analyses.
Conclusions: Here, it is demonstrated that application of transcranial ultrasonography to Neuro-COVID-19 requires a specific adaptation that takes into account brain movements synchronous with breathing as well as the sensitivity of SARS-CoV-2 to ultrasounds.
Keywords: SARS-CoV-2, COVID-19, vaccination, ultrasound, imaging, brain
COVID-19, coronavirus disease 2019; CSF, cerebrospinal fluid; CT, computed tomography; HIV, human immunodeficiency virus; MRI, magnetic resonance imaging; qRT-PCR: real-time quantitative reverse transcription polymerase chain reaction; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2
The term Neuro-COVID-19 refers to the plethora of short- and long-term neurologic and psychiatric manifestations that are frequently associated with infection by severe acute respiratory syndrome-related coronavirus 2 (SARS-CoV-2).1
Involvement of the central nervous system is associated with signs and symptoms that comprise anosmia/ageusia, headaches, dizziness, ataxia, seizures, mental confusion, agitation, cognitive impairment, anxiety, insomnia, delirium, and coma.1 Pathologic findings include brain edema, encephalopathy, myelopathy, encephalitis, meningeal congestion and a wide spectrum of cerebrovascular abnormalities ranging from thrombosis and infarct to hemorrhage.2 Long-term sequelae affect a significant percentage of patients who have recovered. A study from Northern Italy, one of the areas outside China most severely affected by the first wave of the pandemic, showed that, six months after hospitalization, about one third of survivors presented with long-term neuro-psychiatric symptoms that included fatigue, disorders of memory attention and sleep, cognitive impairment, hyposmia, and postural tremor.3 A successive meta-analysis examining 236,379 patients from all over the world yielded comparable results, demonstrating that incidence of neurological or psychiatric disturbances in the six months following the diagnosis of COVID-19 was about 33%.4 Data from this latter study indicate that significant neurological and psychiatric long-term morbidity were independent of the severity of COVID-19.
Neurological and psychiatric manifestations of COVID-19 are attributed to direct invasion of the central nervous system by SARS-CoV-25,1 as well as to indirect effects such as abnormal immune responses with consequent inflammation, and hypoxia associated with respiratory failure.1 However, despite the high incidence of short- and long-term neurological and psychiatric manifestations, studies that investigated for the presence of SARS-CoV-2 in brain specimens from human autopsies in fatal COVID-19, did not yield impressive or unequivocal results.2 Immunohistochemistry with antibodies directed against the viral nucleocapsid or spike proteins, yielded negative results in most cases as if the viral proteins, and hence the virus, were not present, or at least detectable, in those brain specimens; also in situ hybridization for viral RNA yielded negative results. qRT-PCR assays targeting different genes did not add much information since only low levels of signal were identified in frozen or formalin-fixed paraffin-embedded brain tissue specimens.2
The discrepancy between the magnitude of the neurologic and psychiatric symptoms of COVID-19 and the scarcity of evidences demonstrating its presence in the brain could be explained taking into account the existence of reservoirs or sanctuaries for SARS-CoV-2 in analogy with what has been observed with HIV.6,7 The existence of sanctuary sites for SARS-CoV-2 in the brain has the potential to decrease the efficacy of antiviral therapies or vaccination and may even prevent complete eradication of SARS-CoV-2 from the infected organism with the risk of persistence of low-level infection. Low-level infection with associated chronic immune responses and low-level, but persistent, inflammation, may lead to severe long-term consequences; it has already been observed that a significant percentage of recovered patients show a greater risk of developing neurodegenerative disorders long after recovery from COVID-19.8 Therefore, learning from the experience with HIV,9 it is desirable to develop strategies to force SARS-CoV-2 out of brain reservoirs or sanctuaries so that drugs or the immune system can neutralize it.
Since 2017, an original diagnostic and therapeutic procedure aimed at diagnosis and treatment of elusive chronic conditions was described.10 Although this procedure was originally developed for diagnosis and treatment of persistent Lyme, it was realized that it may be useful in other chronic conditions since it is able to evidence the presence of pathogens that otherwise could go undetected. The procedure is composed of a sequence of diagnostic and therapeutic steps that aim at increasing sensitivity and specificity of diagnosis at the same time assessing and optimizing the efficacy of treatments in persistent low-level infections as well as in other chronic conditions associated with low-level inflammation. These goals are achieved by the original exploitation of therapeutic ultrasounds that are used to increase sensitivity and specificity of diagnosis: thanks to the mechanical effects of compression and relaxation at the tissular, cellular and molecular levels, therapeutic ultrasounds force the exit of pathogens from tissue reservoirs or sanctuaries. In this way, they become "visible" to the immune system, to specific drugs, as well as to the specific tools for diagnosis.
Here, it is described how this procedure can be adapted to Neuro-COVID-19 and how application of therapeutic ultrasounds has to take into account the movements of expansion and contraction of the brain that occur in synchrony with breathing. It is also described the potential therapeutic use of ultrasounds in Neuro-COVID-19 since it has been demonstrated that SARS-CoV-2 is sensitive to the range of frequencies used for medical ultrasonography11 and, therefore, may be inactivated by ultrasounds in analogy with what has been observed for other viruses12,13
Transcranial sonography performed to obtain the images presented in Figures 1-3 was performed using an Esaote MyLabFive (Esaote, S.p.a., Firenze, Italy) ultrasound system. This system is approved for several applications that include cephalic - brain - imaging. In the observation reported in Figs 1-3, a linear probe for muscle-skeletal examination (LA 523 by Esaote) was utilized with settings adjusted for adult transcranial imaging as described in Ruggiero et al. This probe has a length of 4 cm and can be used with frequency of 7.5 megahertz (MHz) and acoustic power adjusted to 1.0. As ultrasound transmission medium, Parker Aquasonic 100 gel was used. The probe was applied to the temporal region of the head in correspondence of the squama of the temporal bone. The probe was gently tilted until the hyper-echoic (white) line corresponding to the squama of the temporal bone was horizontal. The results depicted in Figures 1-3 were obtained at the Department of Human Anatomy, Histology and Forensic Medicine of the University of Firenze, Italy, as described in Ruggiero et al. These results are here illustrated, and their significance elaborated, in the context of the object of this article. Figs. 1 and 3 are reproduced from Ruggiero et al.14 under the terms allowed by Firenze University Press. The procedure involving therapeutic ultrasounds can be performed with portable systems such as, for example, the Chattanooga Intelect TranSport Ultrasound system, a system that is commonly used for clinical electrotherapy in the context of physical therapy. These systems have a small round head emitting ultrasounds; this shape is particularly suited for therapeutic application of ultrasounds. In these system, ultrasounds are generated by means of vibration of a crystal and they are transmitted to the chosen part of the body through the aluminum surface of the head and the ultrasound transmission gel. In most systems, therapeutic ultrasounds can be generated with a frequency range between 1 and 3.3 MHz. The procedure for application of therapeutic ultrasounds has been described in detail in Klinghardt and Ruggiero,10 and Antonucci et al.15 For the purpose of this article, in the context of Neuro-COVID-19, the steps concerning the use of diagnostic ultrasonography and therapeutic application of ultrasounds, that is steps 2 and 3 as reported in Table 1 of Klinghardt and Ruggiero,10 are described with particular reference to the temporal lobe of the brain.
Step 2 of the procedure consists of diagnostic ultrasonography that has the role of refining the diagnostic hypotheses put forward by clinical examination as described in detail in Klinghardt and Ruggiero.10 In case of Neuro-COVID-19, transcranial ultrasonography of the temporal lobe of the brain has the primary role of early identification of the neuropathological findings of COVID-19 that are constituted by edema, meningeal congestion, hemorrhages, infarcts, and hematomas.2 The methodology for transcranial ultrasonography described in Ruggiero et al.,14 and in Bradstreet et al.,16 is particularly suited for this scope as it allows detailed visualization of brain structures as demonstrated in Figures 1 and 2. Figure 1 shows ultrasound visualization of normal meninges and temporal cortex. The meninges show the appearance of a structured array of layers with alternating hyper- and hypoechoic lines. The sub-arachnoid space shows the appearance of a horizontal “track”. An anechoic linear space (black in the figure) is paralleled by two hyper-echoic (white) stripes (Figure 1C). The inner anechogenicity of this structure is due the presence of a minute film of liquor that appears black in ultrasonography and shows a thickness of 0.2 mm. The sub-arachnoid space is situated parallel to the temporal squama, and signals the border between the meninges and the cortex. Thickness of the sub-arachnoid space in its entirety was around 0.6 mm (not shown). The thickness of the temporal cortex was calculated by the program of the Esaote machine in 3.8 mm (Figure 1B). Such a thickness was indirect confirmation of the anatomical position of the probe in correspondence of the areas of the temporal cortex that are designated TG and TE (see Figure 1, panel A for anatomical reference). These are the areas involved in the control of the movements of the eyes and in maintaining balance while standing (area TE), as well as in the control of social behavior, mood and decision making (area TG). It is worth noticing that these areas of the temporal lobe are among those that show alterations in Neuro-COVID-19.17 The high degree of spatial resolution enabled visualization of sub-millimetric structures (Figure 2A), that correspond to the neuronal layers of the cortex as represented in Figure 2B.18,19 The hyperechoic structures correspond to irregular cytological architecture that is characteristic of those layers where there is high density of neurons whose soma have irregular shape. The echogenic profile depicted in Fig. 2 A, is superimposable to that obtained by Nissl histologic staining, a classical method to visualize the soma of brain neurons. It is worth noticing that most of the studies on neuroimaging in COVID-19 were performed using computed tomography (CT) or magnetic resonance imaging (MRI),20 two techniques that are expensive, time-consuming, have to be performed in hospitals or specialized structures, involve the use of electromagnetic radiations (ionizing radiations in the case of CT), and cannot be performed in the comfort of the patient's home using portable, low-cost machines. Therefore, given the amount of information obtainable with transcranial ultrasonography, and considering the obvious advantages of this technique, it is proposed to incorporate transcranial ultrasonography in the study of Neuro-COVID-19 either as an independent exam or integrated in the procedure described in Klinghardt and Ruggiero.10

Figure 1 Transcranial ultrasonography of the temporal lobe of the brain
A. Cytoarchitectonic map from Constantin von Economo's original work. Constantin von Economo. Der Zellaufbau der Grosshirnrinde und die progressive Cerebration. Ergebnisse der Physiologie. 29, 1, 83-128. 1929. doi:10.1007/BF01942021, Public Domain, https://commons.wikimedia.org/w/index.php?curid=3640121.
B. Ultrasonographic appearance of the meninges, the sub-arachnoid space and the temporal cortex. Thickness of the cortex of the temporal lobe is consistent with that of the TE and TG areas.
C. Focalization on the sub-arachnoid space. An hyperechoic layer, hypothetically corresponding to a cortical neuronal layer of 0.5 mm is evidenced in the depth of the temporal lobe.
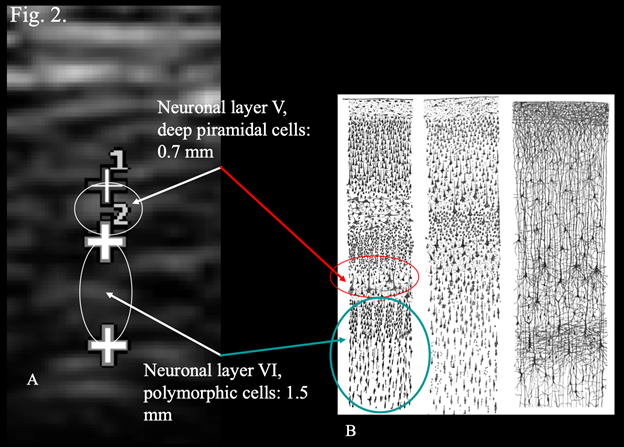
Figure 2 Correspondence between layers of transcranial ultrasonography and drawings of the cerebral cortex.
A. Magnification of areas of the temporal cortex using the zoom application of the ultrasound system.
B. Original drawings by Santiago Ramon y Cajal, taken from the book "Comparative study of the sensory areas of the human cortex", (1899) pages 314, 361, 363. Left: Nissl-stained visual cortex of a human adult. Middle: Nissl-stained motor cortex of a human adult. Right: Golgi-stained cortex of a 1 1/2 month old infant. ISBN 9781458821898, Public Domain, https://commons.wikimedia.org/w/index.php?curid=8513016. Looie496 created file. This figure, under the form of a slide, was presented by M.R. at the World Day of Multiple Chemical Sensitivity, Myalgic Encephalomyelitis and Fibromyalgia, Rome, Italy.
Step 3 of the procedure described in Klinghardt and Ruggiero10 consists of application of therapeutic ultrasounds. In case of Neuro-COVID-19, this step has a double function. 1. Force the exit of SARS-CoV-2 from reservoirs or sanctuaries in brain tissue. 2. Neutralize the virus thanks to the disrupting effects of ultrasounds on viral structures with particular reference to spike proteins.11 Here, it is reported how tailoring the procedure described in Klinghardt and Ruggiero10 to Neuro-COVID-19 requires an additional step that consists in taking into account the movements of contraction and expansion of the brain that occur synchronously with breathing. Previous observations14 of brain movements using transcranial ultrasonography evidenced the known pulsatile movements synchronous with the cardiac cycle as they were reported in studies using MRI.21 In addition to the movements associated with the cardiac cycle, rhythmic movements of contraction and expansion associated with the respiratory cycle were observed. Fig. 3, shows the total thickness of the meninges and the cortex of the temporal lobe at the end of forced voluntary exhalation (Figure 3A, thickness 7.3 mm), or at the end of forced voluntary inhalation (Figure 3B, thickness 6.7 mm). These observations are consistent with the results reported by Turner et al.22 who, using f-MRI, described similar rhythmic pulsations of the brain associated with the respiratory cycle. These movements of contraction and expansion of the brain are of significant magnitude and are to be considered the respiratory equivalent of the Monro-Kellie doctrine that concerns the cardiac cycle and is responsible for cerebrospinal fluid (CSF) flow.23 However, at variance with the movements associated with the cardiac cycle, the movements of the brain associated with the respiratory cycle can be controlled voluntarily as demonstrated in Fig. 3. Therefore, in the context of exploiting the effects of ultrasounds in Neuro-COVID-19, it is proposed to apply the therapeutic ultrasounds every other voluntary exhalation when the thickness of the meninges and cortex is maximal and the movement of expansion of the brain has reached its apex. The rationale for such a timed approach consists in the evident greater hypoechogenicity of the brain structures at the end of forced voluntary exhalation (Figure 3A); such an ultrasonography finding corresponds to a greater amount of extracellular liquid that in turn translates in reduced ultrasound velocity, thus maximizing the mechanical disruptive effects of the ultrasound waves.24 The rationale for applying bursts of therapeutic ultrasounds alternatively, at end of every other exhalation, consists in the observation that expansion of the brain corresponds to increased removal of CSF.21 The burst of therapeutic ultrasounds is aimed at forcing the exit of SARS-CoV-2 from reservoirs or sanctuaries at the same time mechanically disrupting its molecular structures; during the following respiratory cycle, the virus and its debris would be removed by the flow of CSF and then the cycle could be repeated. In addition, it has to be considered that transcranial ultrasounds show anti-inflammatory and anti-edema effects,25 and ultrasounds may favor CSF drainage in analogy with their known effects on lymphatic drainage.26
Here it is demonstrated that transcranial ultrasonography and therapeutic ultrasounds, either incorporated in the procedure described in Klinghardt and Ruggiero,10 or used independently, may prove effective in the integrated diagnosis and treatment of Neuro-COVID-19. The procedures described here are safe, inexpensive, easy to be performed, and can be implemented in the comfort of the patient's home or in any other environment whether in the developed world or elsewhere since they do not require sophisticated instruments or dedicated health structures. Transcranial ultrasonography may help with the early diagnosis of brain lesions, possibly before the onset and development of the severe symptoms of Neuro-COVID-19. Application of therapeutic ultrasounds using the timed procedure described in this article may constitute a significant addition to specific therapies and may maximize the long-term efficacy of immunization at the same time reducing the risk of long-term sequelae of COVID-19.
The author acknowledges the great work of Dr. Aldo Ruggiero, MD (1923-2006), pioneer of radiology in Prato, Italy, founder of the Studio Radiologico Ruggiero, source of boundless inspiration for this and many other scientific articles.
The author contributed solely to the work.
The author declares that he has no conflicts of interest concerning the topics described in this article.
Not applicable. This article does not report observations or experiments performed on humans or animals. The results depicted in Figures 1-3 were previously published in Ruggiero et al.14 These results are here illustrated, and their significance elaborated, in the context of the object of this article.
Not applicable.
Figures 1 and 3 are reproduced from Ruggiero et al.14 under the terms allowed by Firenze University Press (CC BY 4.0).
A pre-print version of this article was posted on Research Square https://www.researchsquare.com/article/rs-776630/v1
Not applicable.

©2022 Ruggiero. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.