International Journal of
eISSN: 2574-8084


Research Article Volume 6 Issue 4
1GenesisCare, St Andrews Hospital, South Australia
2GenesisCare, Fiona Stanley Hospital, Western Australia
3GenesisCare, Bundaberg Base Hospital, Australia
4GenesisCare, The Wesley Medical Centre, Australia
5GenesisCare, Mater Hospital, Australia
6Northern Clinical School, University of Sydney, Australia
7GenesisCare, St Vincent's Hospital, Australia
8Melanoma and Skin Cancer Trials Group (MASCTG), Australia
9University of Technology, Australia
Correspondence: Gerald B Fogarty, St Vincents Hospital, 438 Victoria Street, Darlinghurst NSW 2010, Australia, Tel +61 2 8302 5400, Fax +61 2 8302 5410
Received: June 30, 2019 | Published: July 9, 2019
Citation: Potter A, Price M, Papworth D, et al. A technique for treating extended skin field cancerisation using volumetric modulated arc therapy. Int J Radiol Radiat Ther. 2019;6(4):111-119. DOI: 10.15406/ijrrt.2019.06.00230
Patients with extended skin field cancerisation (ESFC) can have poor oncological outcomes and poor quality of life. The durability of traditional non-surgical skin field treatments is disappointing. Volumetric modulated arc therapy (VMAT) is a newer radiation technique that enables the treatment of large convex skin fields with a homogenous dose in one field of treatment. We set out to describe a VMAT technique used in the treatment of ESFC. The description of this technique discusses patient selection and assessment, history taking and comorbidities, drug therapies known to impact radiosensitivity, and strategies for dealing with in-field invasive disease. Planning advice on positioning, immobilization and scanning at simulation, along with hints for contouring, dealing with organs at risk (OARs) and VMAT arc angles, is provided. Follow-up strategies and areas of future research are also discussed. This report is the precursor to future articles from our group outlining our initial experience of VMAT in ESFC for oncological, functional and cosmetic outcomes.
Keywords: skin neoplasms, radiotherapy, actinic keratosis, Bowen’s disease, in situ squamous cell carcinoma, basal cell carcinoma, volumetric modulated arc therapy, skin field cancerisation, review
AK, actinic keratosis; BCC, basal cell carcinoma; BD, Bowen’s disease; CLL, chronic lymphocytic leukaemia; cm, centimetres; CBCT, cone beam computed tomography; CR, complete response; CT, computed tomography; °C, degrees Celsius; DVH, dose volume histogram; e.g. for example; ESFC, extended skin field cancerisation; Gy, Gray; HIV, human immunodeficiency virus; IEC, intraepithelial cancer; IVD, in vivo dosimetry; KA, keratoacathoma lesions; MRI, magnetic resonance imaging; OAR, organs at risk; mm, millimetres; RT, radiation therapy; RO, radiation oncologist; SSD, source surface distance; SIB, simultaneous integrated boost; SXRT, superficial radiotherapy; 3DCRT, three-dimensional conformal radiotherapy; 3DP, three-dimensional printing
The incidence of skin cancer is rising globally, and Australia has one of the highest incidences in the world.1 The development of new skin cancers can accelerate with age, particularly in the immunosuppressed.2 Patients can suffer from extended skin field cancerisation (ESFC) defined as a field over 50 cm.2,3 These fields harbour pre-invasive cancer, especially actinic keratosis (AK), Bowen’s disease (BD) and intraepithelial cancer (IEC), from which new invasive cutaneous basal (BCC) and squamous cell carcinoma (SCC) can arise.4,5 Poor oncological outcomes and poor quality of life can result.6 Comorbidities can preclude other treatments, especially complex surgery, which may be declined due to fear of an undesirable functional or cosmetic outcome or surgical fatigue. The durability of traditional non-surgical skin field treatments is disappointing, and recurrence is common.7−9 A recent randomised trial by Jansen et al.10 showed that 5% fluorouracil cream was superior to 5% imiquimod cream, methyl aminolevulinate photodynamic therapy (MAL-PDT), or 0.015% ingenol mebutate gel; however, only 75% of patients with a field size of 25 to 100 cm2 on the head maintained a complete in-field response (CR) at one year.
The use of radiotherapy (RT) to successfully treat ESFC has been documented. Barta et al treated a 66-year old with a 10x8 cm scalp AK with 28 Gy given twice weekly in 4 Gy fractions using superficial radiotherapy (SXRT) with a focus-skin distance of 30 cm (25 mA, 29 kV) and a half value layer of 0.3mm aluminium. At 14 months, the CR was maintained.11 Dinehart et al.12 treated 16 patients with conformal megavoltage radiotherapy (3DCRT) with 40 to 60 Gy and achieved in-field control which continued in 13 patients at four weeks. Of 10 evaluable patients at one year, nine had an ongoing CR.12 Pipitone et al.13 treated one patent with extensive scalp recurrence with 3DCRT 60 Gy in 30 fractions and reported an enduring CR at 18 months.13 Despite such favourable results, RT is however not regarded as first line treatment but rather as salvage, which may explain why RT was not mentioned in the 2012 Cochrane review on interventions for actinic keratoses.9
ESFC is usually found on sun exposed areas, including the scalp, forehead, cheeks, forearms, legs, chest, upper back and shoulders. These areas are usually convex to an incident radiation beam. Traditional RT often requires field junctioning and “feathering” which introduces dosimetric uncertainty.14 Newer radiation techniques, like volumetric modulated arc therapy (VMAT), allow treatment of large convex skin fields with a homogenous dose in one field of treatment, theoretically solving the dosimetry problem.3 The use of VMAT in ESFC has been previously published.15,16 This research report set out to describe a VMAT technique for the treatment of ESFC. Planning advice and future research perspectives are also discussed.
Aim of treatment
VMAT in ESFC is curative and aims to provide:
Patient selection and assessment
Previous in-field histology that has confirmed invasive cancer, for example: SCC or in situ disease such as AK, BD or IEC, is helpful in establishing treatment rationale, but a clinical diagnosis without biopsy may be acceptable. Several symptomatic skin fields may be present. Patients can suffer from surgical fatigue with this being the reason they elect to consider RT.
History taking
The radiation oncologist (RO) should undertake a thorough history including the number, site, histology and treatment of any previous invasive lesions within the field. Comorbidities will affect a patient’s ability to cope with radical intent treatment and must be carefully elicited. Prior skin and nodal surgery, for example, can predispose patients to lymphoedema during treatment. Questioning current and previous drug therapy is important as some drugs, such as methotrexate, hydroxy urea, and some long-term antibiotics, can cause increased radiosensitivity.17 Immunosuppression must be also be considered.
Age
A recent literature review of carcinogenesis in RT treated skin found that modern RT is associated with a risk of one cancer per one thousand persons treated for every ten years of life lived after treatment. The resultant cancers are usually BCC.18 This risk needs to be weighed up against the risk of invasive cancer from untreated ESFC, especially in younger patients.
Examination
The field in question needs to be thoroughly examined for active invasive lesions that may require a higher dose of RT or another treatment modality (e.g. excision). In situ tumour bulk may be better appreciated by palpation rather than inspection. Some thickness may be due to scarring or lymphatic blockage due to prior therapy. Pre-existing lymphoedema, especially in association with previous scars, should be noted as it can flare during RT. Finally, patients intending to have multiple sites (e.g. bilateral areas on the face) treated concurrently should be carefully assessed as toleration may wane.
Consent
Informed patient consent needs to cover the possibility of acute RT side effects, such as wet desquamation and lymphoedema, especially in a dependent limb. The risk of mucositis in nearby organs (e.g. nose, facial sinuses) should be mentioned, as should potential side-effects to organs in the exit and entrance paths of the VMAT beams (e.g. lacrimal glands, salivary and thyroid glands, lens, brain etc).
Allied health review
Prior to simulation, a dental review for patients with their own dentition and salivary tissue in the integral volume is reasonable, as is review with a dietitian if any part of the digestive tract is in the radiation volume.
Nursing care and review during treatment
Weight maintenance is essential to avoid variation in contour and anterior/posterior separation during VMAT RT, and weekly weighing is an important nursing function. Nurses should also ask patients receiving arm treatment to remove any rings due to the risk of swelling. This is particularly true for patients who are on dialysis, have arteriovenous fistulas, or who have had previous axillary lymphadenectomy.
Patient positioning
Positioning needs to ensure that untargeted body parts will not be in treatment of arcs. For lower legs, the leg being treated should be in extension with the contralateral limb flexed at the hip and knee, and the knee positioned outside the entrance and exit beams of the arc (Figure 1). If this is not possible, the legs should be separated, and the limb being treated should ideally be elevated to maximise the angles that the arc can deliver without entry or exit beams into the contralateral limb (Figure 2). For arms, the patient can adopt the “superman” position. Lying prone on the treatment couch, the arm for treatment is extended above the head. The contralateral arm is positioned by the patient’s side, out of the beam path (Figure 3). Forearms are best positioned pronated as the usual field of ESFC is on the dorsal aspect of the arm. This also enables bolus to be more easily applied. Care should be taken to ensure that the most proximal filed border is superior to the patient’s head. If this is not the case, the exit of the VMAT arc will supply a low dose of radiation to the patient’s brain. Where the proximal border results in a field extending into the head, or where the patient’s condition contradicts the use of the superman position, the arm to be treated can be positioned pronated by the patient’s side. When both limbs are being treated, a treatment position reset is needed mid fraction. Immobilisation of the whole patient needs to be considered. Head and neck fields require a personalised thermoplastic mask (Klarity Medical Products, Newark OH). Limbs and torso should be positioned in a vacuum bag.

Figure 1 Positioning at simulation needs to ensure that other body parts will not be in the treatment arcs. For example, when treating a lower leg, the leg for treatment is in extension, the other limb is flexed at the hip and knee to be outside the entrance and exit beams of the arc.

Figure 2 If retraction of the leg is not possible, for example when treating both legs, the legs are separated. (A) The limb can even be elevated. (B) So as to maximise the angle around the leg that the arc can treat.

Figure 3 For arms, the patient can adopt the “superman” position. The patient lies prone on the treatment couch with one or both arms for treatment extended above the head. For one arm, the other arm is by the patient’s side, out of the way of the beam.
Simulation
The RO should use a removable marker to outline on the skin the symptomatic area as the clinical target volume (CTV) (ICRU 50).19 The drawn lines are copied onto plastic sheets as templates which are then stored electronically in the patient’s medical record so that treatment fields can be reproduced in the future. This is important to determine if any future recurrence is in field, at field edge, or out of field, with salvage therapy in mind. Wires should then be placed over the RO’s marks so that the CTV can be captured during the planning CT scan. The CTV contour is vital for expansion to the planning target volume (PTV).
On the lower leg, the CTV wires should leave a skin strip of at least 2.0 cm width to enable adequate dermal lymphatic drainage. There is controversy around whether this is needed for the upper leg and arm, and indeed whether it is necessary in the leg for those who have circumferential disease. Clinically invasive lesions within the field need to be individually wired and contoured as gross tumour volumes (GTVs) (ICRU 50),19 numbered sequentially, and noted. The GTVs can be treated with a simultaneous integrated boost (SIB) or a two-phase approach. At simulation, detailed photos should be taken of the treatment set up.
Involvement of the patient in defining the area to be treated
For head, neck and chest lesions, a face mirror can help patients engage in the mark-up procedure. It is important to include areas from which previous cancers have been removed. The agreed treatment area may not cover all visible disease, particularly if the RO decides that treatment of all diseased areas may cause too much acute toxicity.
In vivo dosimetry (IVD) planning
The points of interest to measure dose with IVD should be marked on the area for treatment by the RO at simulation, photographed and wired. The wires capture the marks on the planning scan.
Bolus
As VMAT uses skin sparing megavoltage (MV) x-rays, the use of bolus is essential to ensure full dose to the skin. Bolus (usually 1.0 cm thickness) is applied prior to scanning and should project peripherally to at least 2.0 cm outside the wired area.
For the scalp, a minimum of 8.0 mm of thermoplastic bolus is taped underneath the thermoplastic mask prior to scanning. For limbs, 10 mm of gel bolus or Superflab® (Radiation Products Design Inc, Albertville MN) can be used. A crepe bandage can hold the bolus in position. Tubigrip® (Mölnlycke Healthcare, Gothenburg, Sweden) may be used on the leg (Figure 4). Air gaps beneath the bolus can result in approximately 10 percent dose variation/uncertainty at the surface.20 The planning CT scan should be inspected for the presence of air gaps. For the nose, the air gap between bolus and skin should not exceed 2.0 mm; for scalps and limbs the maximum should be 5.0 mm. If the above tolerances are not met, the bolus must be adjusted. The scan should be repeated until placement is satisfactory.

Figure 4 For lower legs,10mm of gel bolus or Superflab® is applied. Tubigrip® can be used to hold the bolus in position.
Planning computed tomography (CT) scan
The planning CT scan is performed in the treatment position at an average of 2.0 mm slices. Nose plans, scalps and limbs should preferably have a maximum slice thickness of 1.0 mm, 2.0 mm, and 2.5 mm, respectively. The extent of the scan needs to be at least 5.0-7.0 cm proximal and distal to the lesion/region border for accurate dosimetry. Scanning needs to be extend above and below the area of interest to contour whole organs at risk (OARs) to ensure a representative dose volume histogram (DVH), which may include the lungs, lacrimal glands and hippocami etc.
Contouring
When contouring in situ disease, the maximum depth of skin appendages is 4.5 mm,21 so the CTV should be to a depth of 5.0 mm.
Target volumes are:
As the resulting PTV is quite thin, it mandates that pre-treatment set up and image verification is done meticulously, preferably with daily cone beam CT (CBCT) (Figure 5). A GTV may be contoured when using SIB, or a two-phase technique, to treat macroscopic invasive disease within the area of ESFC. OARs are contoured, and any automatically generated contours need to be checked for veracity.


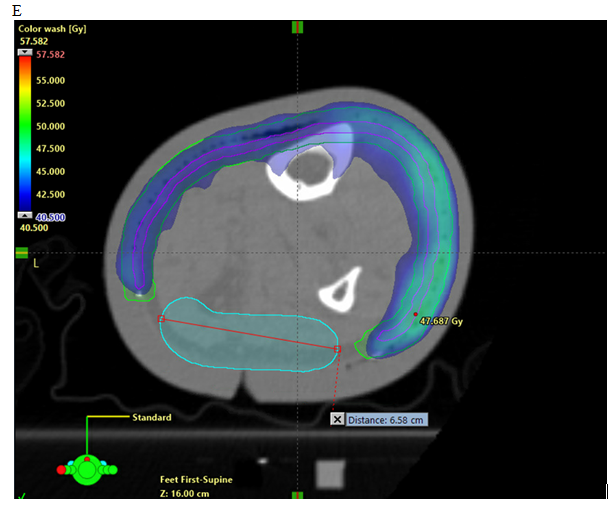
Figure 5 Axial planning CT of a leg showing. (A) Contouring of CTV. (B) Contouring of PTV and strip of skin as an OAR. (C) Addition of a deep “skin avoid” structure which has a dose limitation of mean less than 25Gy. (D) Isodose line dosimetry showing decreased dose to strip (18 - 20Gy isodose line should not be overlapping the skin sparing strip). (E) Same as in D showing dosimetry in dose wash set at 40.5 Gy.
Radiation dose prescription
The doses used successfully in our prospective series are in Table 1. These will be applied in prospective studies in the absence of clear dose sensitivities of in situ disease.22 The use of a mid-treatment break (“split course” schedule) for limbs is controversial. Some practitioners argue that in situ disease is susceptible to accelerated repopulation and that it should be treated without a break.23 Others feel that a break has no impact on the required total dose or clinical outcome, but that it is necessary to avoid unacceptable short-term toxicity (e.g. pain and swelling), which may affect total dose delivery. Our practice is to mandate a two-week treatment break between fraction 10 and 11 of a 25-fraction course for lower legs.
|
Total dose (Gy) |
No of fractions/weeks of RT |
Dose per fraction |
BED* early |
BED**late |
EQD2*** α/β =10(Gy) |
EQD2*** α/β =3(Gy) |
|
For in situ disease |
||||||
|
45 |
25/5-May |
1.8 |
53 |
72 |
44 |
43 |
|
50 |
25/5-May |
2 |
60 |
83 |
50 |
50 |
|
50 |
20/4-Apr |
2.5 |
63 |
92 |
52 |
55 |
|
For invasive disease (SIB) |
||||||
|
55 |
25/5-May |
2.25 |
69 |
98 |
56 |
58 |
|
60 |
30/6-Jun |
2 |
72 |
100 |
60 |
60 |
Table 1 Biologically effective dose (BED) for ESFC therapy29,30
*Biologically effective dose of early responding tissue using α/β of 10
**Biologically effective dose late responding tissue using α/β of 3
***Equivalent dose in 2Gy fractions (EQD2)
(Biologically effective dose (BED)=n x d(1+d/α/β)
n=number of treatment fractions
d=dose per fraction in Gray (Gy)
α/β=dose at which the linear and quadratic components of cell kill are equal;
Equivalent dose in 2Gy fractions (EQD2)=D x {d+(α/β)}/{2+(α/β)}
EQD2=dose delivered in 2Gy fractions that is biologically equivalent to a total dose
D=total dose given in Gy
d=dose per fraction in Gy
α/β=dose at which the linear and quadratic components of cell kill are equal
SIB-simultaneous integrated boost
Our approach is to give a dose of 45 Gy in 25 fractions to in situ disease with SIB to 55 Gy. For areas of invasive carcinoma, a phase two technique to a total dose of 60 Gy is administered. Macroscopic disease lacking response at the end of treatment may need a further 10 Gy boost in two 5 Gy fractions. Limbs with vascular insufficiency have difficulty healing and standard fractionation with 2 Gy per day, or even slight hyper fractionation with 1.5-1.8 Gy per day, is advisable. Clinical dose studies for in situ SCC are needed. Dose limits are set for OARs (Table 2).
|
Treatment site |
Dose & fractionation scheme |
Organ |
Dose goal - primary |
Dose goal – variation acceptable |
|
Scalp |
Scalp 45-50Gy/25# ± SIB boost 55-60Gy/25 fractions |
Brain |
Mean D < 8Gy |
Mean D 8-12Gy minor deviation |
|
Lens |
Max < 6Gy |
|
||
|
Lacrimal gland31 |
Max < 30Gy Mean D < 25Gy |
|||
|
Normal Tissue |
ALARA |
Table 2 Organs at risk
Beam parameters
For VMAT plans, the beam arrangement should be with two 6MV full/partial arcs. Arc lengths are selected to avoid dose to normal tissues.
Normalisation and plan evaluation
Plans should be normalised to satisfy the ICRU 83 guideline.24. The recommended normalisation and reporting values include:
Plan acceptance
The therapeutic dose will extend beyond the CTV, which can impact nearby hair bearing areas. The number of arcs required depends on dose homogeneity and minimising dose to OARs. VMAT gives a uniform dose through the treatment volume and has none of the “top down” problems associated with fixed source external beam solutions.18
Daily set up and verification imaging
As the PTV is thin (often just 1.0 cm thick), there is a risk of ‘geographical miss’ if adequate care is not taken during set up. During treatment, the area being treated may swell, especially if on a dependent limb. The dose may then be imparted to the dermis, worsening the swelling. There is less chance of this happening if CBCTs are used rather than just matching to bone with standard kilovoltage (KV) imaging. Any circumferential swelling of greater than 5.0 mm necessitates either a treatment break or re-planning with a newly acquired CT.
Patients should be reviewed weekly by the RO. Tumour lysis (Figure 6) causing skin necrosis is to be expected relatively early during treatment as the cancer dies. Toxicity scoring can be difficult as extensively affected skin may show signs of breakdown at relatively low doses of RT, resulting in up-scoring of toxicities. The effect of treatment can be gauged by documenting the changes in normal skin in and around the RT field. Side effects typically start after the period of tumour lysis. Anecdotally, patients with early RT reactions may be radiation sensitive and a smaller dose may be possible for cure. The RO must be prepared to titrate the dose and to stop treatment when early wet desquamation of in-field normal cells is observed. Pain is usually responsive to paracetamol and opiates are rarely required. Pain in the lower limbs can be helped by compression stockings and elevation.
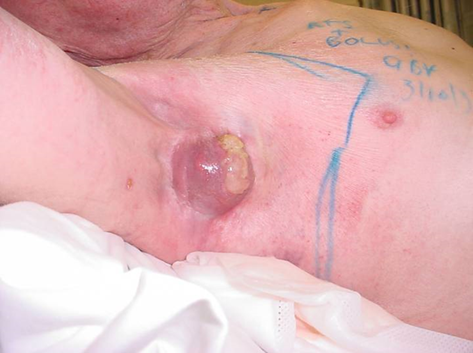
Figure 6 Differing radiation sensitivities of normal versus tumour cells. Definitive treatment of ulcerated and bleeding axillary metastases of SCC. Tumor lysis is shown in the yellow mass of necrosis that sits above the mass that is dead cancer already apparent at 27 Gy. Note that there is a bolus covering normal in-field skin and so it is also receiving full dose.
Follow up
Follow up should be undertaken within a multidisciplinary approach. If required, radiation or community-based nurses should be involved in daily dressings and photos can be sent electronically to assess problems. Rarely, these can include cellulitis, or prolongation of the acute reaction, and may be due to concurrent medication.
Special treatment considerations
Limbs: After doses as low as 18 Gy, patients having lower leg treatment may experience pain due to the acute inflammatory process occurring within the skin. To ensure treatment completion, we mandate a treatment break of at least two weeks, especially for the lower legs. Dose constraints are in Table 3. Preliminary experience indicates this inflammatory response may also have an anti-tumour affect that could be mediated by hyperthermia as low as 39°C.25
|
Treatment site |
Dose & fractionation scheme |
Organ |
Dose goal - primary |
|
Limbs |
Limbs 45Gy/25# ± SIB boost 55-60Gy/25 fractions |
Lymphatic protection / Avoidance of lymphoedema |
No mean dose data available. Recommend 18-20Gy isodose line should not be overlapping the skin sparing strip. 1. Limb sarcoma literature suggests leaving a 20% to 25% circumferential preservation strip along a section of the limb where lymphatics are thought to traverse.30 2. Use of a split course technique (mandatory break of at least 2 weeks duration to allow for resolution of acute symptoms) to reduce risk of lymphoedema |
|
Other normal tissue –contralateral leg, torso |
ALARA |
Table 3 Dose constraints for limbs
Scalps: ESFC is quite common in the elderly male experiencing male pattern baldness. As VMAT irradiates more surrounding skin than other techniques, increased alopecia occurs. The patient needs to be warned of hair loss at consent.
Ongoing research into refining the technique
Bolus: Ill-fitting bolus remains a significant challenge. Research is being undertaken on bolus created by three-dimensional printing (3DP) to reduce air gaps between the bolus and skin. 3DP bolus may also decrease set up time. 3DP does, however, involve a separate visit for an additional CT scan to create the 3DP template.
How much can be treated in a single field?: The VMAT technique can, in theory, treat to maximum multileaf collimator extension-around 40x40 cm in a modern linac. The source surface distance (SSD) cannot be increased with VMAT, so this sets the upper limit of field size. More area means that a larger area of skin will suffer acute toxicity, which may be intolerable for some patients.
Active invasive lesions within an area of ESFC: Our experience to date shows that the break we give patients with ESFC only has no effect on late term oncological control.26 For patients presenting with an active invasive lesion within an area of ESFC, our practice is to treat the invasive lesion with SIB to enable more dose per fraction. It may be wise to treat the invasive lesion during any break to prevent progression.
Variable radiation sensitivity: The radiation doses used in ESFC are used in other tumour types. Some patients, however, develop acute side effects at a lower dose than anticipated and stop treatment before the expected dose required for cure. After more than a year of follow-up, it is our experience that in-field control remains. In situ tumour cell radiation sensitivity may be similar to that of normal cells and due to a genotype27 or phenotype problem.28
For patients suffering from ESFC, VMAT is a novel technique which promises a more permanent solution than current non-surgical treatments. In Australia, ROs have developed VMAT skin protocols to ensure reproducible treatment nationwide. This standardisation will assist the design of prospective comparative studies and future clinical trials.
The authors wish to thank Aileen Eiszele BA(Hons), DipEd, GradDipBus, for medical writing and manuscript preparation. They would also like to acknowledge the staff at GenesisCare who made the VMAT technique in ESFC possible, radiation therapy departments across the GenesisCare network for data and image collection, and those patients who consented to being treated with a new technique.
Ethics approval and consent to participate
Ethics approval was not required as this is a description of a treatment technique.
The authors declare that they have no conflicts of interest.
There was no funding provided.

©2019 Potter, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.