International Journal of
eISSN: 2574-8084


Research Article Volume 8 Issue 3
1GenesisCare, Dept of Radiation Oncology, Mater Sydney Hospital, Australia
2Dermatology Department, Melanoma Institute Australia, Australia
3Sydney Melanoma Diagnostic Centre, RPAH and Medical school, The University of Sydney, Australia
4Sydney Eye Hospital, Macquarie St, Australia
5Discipline of Clinical Ophthalmology, University of Sydney, Australia
6Department of Plastic Surgery, St Vincents Hospital, Australia
Correspondence: Professor Gerald Blaise Fogarty, Genesis Care, St Vincent’s Hospital, Victoria St, Darlinghurst 2010, NSW, Australia, Tel +612-8302-5400, Fax +612-8302-5410
Received: May 18, 2021 | Published: July 1, 2021
Citation: Gorjiara T, Conway A, Sullivan J, et al. A technique for external beam radiotherapy to the eyelid without the need for an internal eye shield. Int J Radiol Radiat Ther. 2021;8(3):92-98. DOI: 10.15406/ijrrt.2021.08.00300
Cancer of the eyelids is difficult to treat. Surgery can lead to tissue loss. Superficial radiotherapy (SXRT) can be used in this scenario, but each fraction requires topical ophthalmic local anaesthetic and the insertion of an internal eye shield (IES) to protect the radiation sensitive anterior structures from the incident beam during beam-on time. The use of an IES comes with challenges. A technique was developed to avoid the IES based on the ability of selected cooperative patients to move the anterior structures out of the incident beam during beam-on time.
We present two cases in which this technique was used. Case 1 involves the lower eyelid and case 2 the upper eyelid.
Case 1 was a 69-year old, immunosuppressed woman needing definitive SXRT for lentigo maligna (LM) of the lower eye lid. She was treated using the new non-IES technique to a total dose of 50 Gy in 25 fractions using a 100 kV beam from a superficial Xstrahl 300 radiotherapy unit (Xstrahl, Surrey, UK). In vivo dosimetry (IVD) measured during a fraction on the posterior of the external eye shield located above the anterior structures showed that the transmitted dose through the external eye shield was less than ten percent of the dose applied. The patient developed conjunctivitis during SXRT that responded to topical antibiotics. Ophthalmology review five months post SXRT showed no change in eye function from baseline. Reflectance confocal microscopy (RCM) eight months after SXRT showed no LM.
Case 2 was a 72-year old woman needing post-operative radiotherapy (PORT) for sebaceous cell carcinoma (SebC) of the upper eyelid. She was treated with 60 Gy in 30 fractions at 5 fractions per week with 100kV SXRT using the new non-IES technique. All the treatment was delivered on time. As per Case 1, IVD under the external eye shield showed the transmitted dose was ten percent of the dose applied. Ophthalmology review pre- and post-RT showed no change in eye function. She remains in complete remission six months after the end of PORT.
The new non-IES technique is safer and quicker and simplifies the workflow. This has become the standard technique in our department for treating eyelids with SXRT. Multidisciplinary care involving an ophthalmologist pre- and post-SXRT is advised.
Keywords: superficial radiotherapy, skin cancer, eyelid, internal eye shield, 3D printed external shield
Malignancies of the eyelid are a challenge to treat. Surgery can lead to tissue loss and may result in lid malposition, ectropion, and damage to the ocular surface.1 Definitive radiotherapy (RT) has a high cure rate and conserves tissue.2-4 Modern fractionated radiotherapy (FRT) ensures that fibrosis and dryness are minimised, leading to acceptable functional and cosmetic outcomes.5 Superficial RT (SXRT) can be used in this scenario.6 Each fraction of SXRT traditionally requires an internal eye shield (IES) to protect the radiation sensitive anterior structures from the incident beam during beam-on time. The radiation sensitive anterior eye structures include the cornea, iris and lens.5 The sclera is relatively radiation resistant, even to large fractions of RT.7
IES comes with challenges. Each fraction requires topical ophthalmic local anaesthetic. There is the possibility of damage to the eye on insertion and removal. Patient cooperation is needed. There is also a risk of trauma from the IES handle that protrudes between the eyelids. Other challenges include the cost of the device and the logistics of its sterilisation between fractions. IES can also have a complicated departmental workflow.
A technique for radiotherapy to the eyelid without the need for an internal eye shield
A technique that avoided using the IES during SXRT of the eyelids was developed and named the ‘non-IES technique’. This technique is based on the ability of selected cooperative patients to move the radiation sensitive anterior structures out of the incident beam during beam-on time. This manoeuvre means that the sclera is the only part of the globe under the eyelid that is in the primary beam. The difference between an IES technique and a non-IES technique is shown diagrammatically in Figure 1.

Figure 1B The new non-IES technique for radiotherapy to the eyelid without the need for an IES.
Figure 1 Diagrammatic comparison of the traditional IES technique and the new non-IES technique.
Legends: “A” refers to the anterior chamber of the eye which is RT sensitive. “Globe” refers to the eye globe. The stars indicate upper and lower eyelids. On the right sided eyelid there is a skin lesion requiring SXRT represented as a black rectangle. This is being irradiated by the incident beam shown by the black arrow. Dotted lines show the lateral extent of the beam.
The large black device above the anterior chamber is the IES. Note that the IES rests on cornea, which is the surface of the radiation sensitive anterior chamber. The IES protects the anterior chamber when the eye is in a neutral unrotated position. Note that the IES handle parts the eyelids and protrudes above the level of the eyelids beyond the body contour which needs to be avoided by the patient and RT staff. There is also a risk of collision with the SXRT applicator. If this occurs there is a risk of eye trauma, and a repeat calibration of the treatment set up may be required.
Legends are as above. In this technique, when treating a selected cooperative patient, the anterior chamber is rotated on the globe by the patient to our left, out of the primary beam. The anterior chamber is protected by rotation out of the beam. Only the sclera is in the primary beam. Nothing rests on the cornea except the normal eyelid. There is no need for an IES. The eye is closed, that is, eyelids are together. There is no protruding IES handle.
The typical time to deliver a fraction of SXRT using a 100 kV beam is approximately one minute, depending on patient setup. A cooperative patient can close their eyes and rotate the globe so that the anterior chamber is out of the primary beam. They can comfortably look away for the duration of beam delivery when instructed by treatment staff that beam time has started and stopped.
To further enable this technique, and decrease any dose to the globe, a customised 3D printed external shield was developed. This device enabled adequate coverage of the treatment region while helping to spare the anterior structures. Briefly, a customised shield is made using AttenuflexTM 3D printed material (3Done*, QLD, Australia) based on the delineation made at simulation by the radiation oncologist. AttenuflexTM is a customised flexible sheet and consists of tungsten carbide powder with a biocompatible thermoplastic binder that is 1.6 mm thick. AttenuflexTM is sufficient to absorb more than 99.5% of the original 100 kV beam. The customised shielding is designed in such a way that there is an aperture over the area requiring treatment whilst the anterior structures of the globe under the eyelid are covered (Figure 2).

Figure 2C SXRT field with eye closed. The patient was asked to look superiorly under the closed eyelid. The cornea could be lightly palpated under the superior eyelid. Its approximate position is shown by a black circle indicated by arrows.

Figure 2D Customised AttenuflexTM shielding. This shield lies on the skin and further refines the actual field that is treated. The thick black arrow indicates the superior edge. The thin arrow indicates an extra width of the template that helps to further protect the anterior structures when placed in situ.
Figure 2 Case 1: Lentigo maligna of left lower eyelid.
This paper presents two patients who underwent this technique. The first case had a lesion on the lower eye lid, and the lesion in the second case was located on the upper eyelid. Both patients were reviewed by an ophthalmologist prior to and after SXRT. This multidisciplinary help was invaluable to ensure that the eye was not at risk.
Case 1
Case 1 is definitive SXRT for lentigo maligna (LM) of the lower eye lid. The patient was a 69-year old immunosuppressed woman in a wheelchair with biopsy proven LM of the left lower eyelid. The area was mapped with reflectance confocal microscopy (RCM) and found to be quite large. The patient declined surgery. She was prescribed 50 Gy in 25 fractions to be delivered using a 100 kV beam by a superficial Xstrahl 300 radiotherapy unit (Xstrahl, Surrey, UK). (Figure 2A-D). The Gafchromic EBT3 films (Ashalnd Advanced Materials, NJ, USA) were used to perform in vivo dosimetry (IVD) for this patient. The IVD measured on the posterior of the external eye shield during a fraction showed that the transmitted dose was less than ten percent of the dose applied. The patient developed conjunctivitis during SXRT (Figure 3) that responded to topical antibiotics (Figure 4) by the end of a week following SXRT. Ophthalmology review five months post SXRT showed no change in eye function from baseline. RCM eight months after SXRT showed no LM.
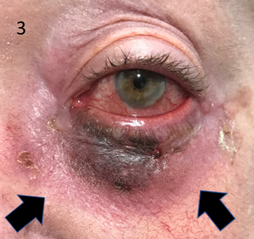
Figure 3 Conjunctivitis during SXRT that responded to topical antibiotics. Note the developing erythema in the treated skin area as indicated by black arrows.
Case 2
Case two describes the use of post-operative radiotherapy (PORT) for sebaceous cell carcinoma (SebC) of the upper eyelid. A 72-year old woman had a lump develop in her left lateral upper eyelid. Histopathology of a wedge resection showed an ellipse of eyelid flesh measuring 8 x 9 x 5 millimetres (mm), extending in thickness from the eyelid skin to the palpebral conjunctiva and bearing a lesion 7 x 7 x 1 mm. Microscopy showed a fully excised SebC with the closest margin at 1.1mm. The hospital multidisciplinary meeting recommended PORT. Discussion with other radiation oncologists suggested at least a 10mm margin on the scar (Figure 5).

Figure 5A Left eye SXRT planning - eyelid closed. The long thin vertical arrow indicates a scar while the short diagonal arrows show the inferior borders of the SXRT field.

Figure 5C Left eye SXRT planning. Eyelids open with the eye moved to the patient’s right and away from the beam. The superior arrow shows the most medial extent of the SXRT field. The inferior arrow shows a mark on the skin which was the most lateral extent of the anterior structures. This mark was made when the cornea was lightly palpated through the eyelid when the eyelid was closed and moved to the right. This is also the most lateral extent of the anterior structures when the eye is open and moved to right, as shown in this figure.
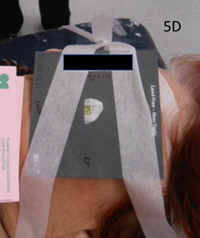
Figure 5D Left eye SXRT planning from the lateral position showing treatment set up with customised AttenuflexTM SXRT shield in place for treatment.

Figure 5E Close up of left eye SXRT planning from the anterior view. IVD under the medial edge of the shield (which would be over the anterior structures – see star) showed less than 10% dose transmission.
Figure 5 Case 2 at planning.

Figure 6B At 50 Gy. Eye open and moved to right showing no conjunctivitis under where the beam would have irradiated the sclera.
Figure 6Case 2 at 50Gy.
The patient was treated with 60Gy in 30 fractions at 5 per week with 100kV SXRT using the new non-IES technique. IVD measured with the same dosimetry films on the posterior of the external eye shield during a fraction showed that the transmitted dose was less than ten percent of the dose applied. All treatment was delivered on time. The patient reported some discomfort due to dryness at four months, and this was treated with punctal plug and lubricants and fully resolved. Ophthalmology review pre- and post-RT showed no change in eye function.
We describe a RT technique which avoids the daily insertion of an IES in patients undergoing RT to the eyelid. This non-IES technique was used in two patients who required fractionated RT. The first case had a lesion treated definitively on the lower eye lid. The second involved a lesion treated in the post-operative or adjuvant scenario on the upper eyelid. We now routinely use this technique in selected cooperative patients.
The new non-IES technique, as compared to the IES technique, causes tissues to be exposed differently to the radiation beam. The palpebral conjunctiva is the inner layer of the eyelid that moves against the globe. In the IES setup, the eyelid is separated from the globe by the IES and only the palpebral conjunctiva is irradiated. With this new technique, however, both the palpebral conjunctiva and the bulbar conjunctiva, the latter which covers the surface of the sclera, are irradiated. This increases the chance of developing acute RT effects towards the end of fractionated treatment due to acute inflammation of the tissue. Theoretically, this increases the risk of RT-induced conjunctivitis.
IES comes with challenges. There is the possibility of damage to the eye on insertion and removal, especially when the eyelid is tight, as may be found in patients with glaucoma or exophthalmos. There is also the possibility of damage to the eye through trauma, or through the introduction of infection. Skilled radiation therapists are needed. Patient cooperation is paramount to insert the IES and the patient must be able to tolerate its position between the eyelid and globe while RT is administered. There is a risk of trauma. The IES has a handle on it that protrudes between the eyelids beyond the body contour. This is a potential source of eye trauma if it is inadvertently knocked by the SXRT applicator, external eye shield, staff, or patient. The risk increases as treatment progresses and as the eye becomes more inflamed and the tissues more friable. Other challenges include the cost of the device. Usually several are need as it is not possible to ensure that a single IES will be sterilised in time for the next daily fraction. Some IES also need special sterilisation and can be damaged if the appropriate care is not carried out by the hospital’s central sterilisation unit.
IES can also create a more complicated departmental workflow due to care pathways. Patient care is transferred multiple times between the different radiation craft groups. On each visit, a nurse applies anaesthetic drops into the eye being treated in preparation for the IES. Care is then transferred to experienced radiation therapists who place the IES and deliver treatment. Patients undergoing treatment of the eyelid are often old and have comorbidities, such as keratitis, that can affect the insertion of the IES. Therefore, in some departments, the protocol requires a radiation oncologist (RO) to insert the IES, mandating another transfer of care. After placement of the IES, the patient is then positioned, and radiation is administered by the RTs. After treatment, the RTs or RO removes the IES and the patient’s care is transferred back to nursing. A nurse applies an eye patch so that the eye is protected from injury while insensate. Some departmental protocols require the patient to remain in the department until the local eye anaesthetic wears off and the eye regains normal sensation.
These points of care are summarised in Table 1. For each treatment, there are eight points of care for the IES technique versus three for the new non-IES technique. Table 2 details a comparison between the two techniques. In summary, with the IES technique, a patient’s care in the department is more complicated, and is longer than their treatment time, and this has implications for treatment bookings, the patient's treatment experience and, in these times, COVID safety measures. Providing safe and effective RT without the need for an IES provides significant advantages for all involved craft groups as well as for patients.
IES technique |
Additional time |
Non - IES technique |
||
Point |
|
|
Point |
|
1 |
Pt arrives |
|
1 |
Pt arrives |
2 |
Nurse – LA |
15 and handover to RTs |
|
|
3 |
RT/RO Insertion of IES |
15 |
|
|
4 |
Treatment |
|
2 |
Treatment |
5 |
RT/RO Removal of IES |
10 and handover to nursing |
|
|
6 |
Nurse – Eyepatch |
10 |
|
|
7 |
Wait in department until sensate |
60 |
|
|
8 |
Discharge |
|
3 |
Discharge |
Total points |
8 |
110 – (i.e. one hour and 50 minutes); at least 2 handovers |
|
3 |
Table 1 Comparison of departmental workflows between the IES and non-IES techniques
Asterix – based on approximate times in our department including handover
Abbreviations: LA, insertion of topical ophthalmic local anaesthetic; Pt, patient; RT, radiation therapist; RO, radiation oncologist; IES, internal eye shield.
|
IES |
Non IES |
Operational issue |
|
|
Need for a select cooperative patient |
Yes |
Yes |
Increased risk of conjunctivitis due to |
No |
Yes |
Need for clear working relationship with |
Yes |
No |
Cost of multiple IES devices |
Yes |
No |
|
|
|
Insertion |
|
|
Need for skilled RTs or RO to insert |
Yes |
No |
Danger of corneal abrasion on insertion |
Yes |
No |
Increased risk of trauma with tight eyelid |
Yes |
No |
|
|
|
When IES in place: |
|
|
Increased risk of trauma due to IES handle |
Yes |
No |
Increased risk of trauma as RT progresses |
Yes |
No |
More risk of damage to insensate eye |
Yes |
No |
Need for eye patch over insensate eye |
Yes |
No |
|
|
|
Overall |
|
|
More time spent by patient |
Yes |
No |
More handovers of care |
Yes |
No |
Table 2 Comparison between the two techniques
*110 minutes (i.e one hour and 50 minutes); at least 2 handovers based on approximate times in our department. See Table 1.
IES, internal eye shield; RT, radiation therapist/radiation thearpy; RO, radiation oncologist.
We presented two cases requiring superficial radiation therapy (SXRT) to the eyelid that were successfully treated with a technique that avoids the use of an internal eye shield (IES). The first case had a lesion treated definitively on the lower eye lid while the second case involved a lesion on the upper eyelid that was treated in the post-operative or adjuvant setting. The technique is based on the ability of selected cooperative patients to move the anterior structures out of the incident beam during beam-on time.
The new non-IES technique is safer, quicker and simplifies the workflow compared to using an IES. As more of the conjunctiva is irradiated with the non-IES technique, there is, however, a higher risk of conjunctivitis, but this is reversable and responds well to topical treatments. This new technique has become the standard of care in our department for treating eyelids with SXRT. Multidisciplinary care involving an ophthalmologist pre- and post-SXRT is advised.
The authors wish to thank the patients involved for consenting to have their clinical details and photos used. The authors also thank Aileen Eiszele of A&L Medical Communications for editing the manuscript and for overseeing the journal submission process. Thanks also to Jack Fogarty for the artwork of Figure 1. Recognition and thanks should also be extended to the ever-innovative GenesisCare radiotherapy department at the Mater Hospital, Sydney, Australia.
The authors have no conflicts of interest to declare.

©2021 Gorjiara, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.