International Journal of
eISSN: 2574-8084


Literature Review Volume 11 Issue 5
1Department of Diagnostic Radiology, Nuclear Medicine, King Abdullah Medical City, Saudi Arabia
2Department of Nuclear Medicine, Kings College London, UK
Correspondence: Ateef Aldehlaui, MSC, RT, Nuclear Medicine Senior Specialist, Nuclear Medicine Supervisor- King Abdullah Medical City, Saudi Arabia, Tel +966547337850
Received: October 14, 2024 | Published: October 25, 2024
Citation: Aldehlaui AM, Fowler C, Alzahrani A. A meta-analysis of bone tracer scintigraphy in the diagnosis of cardiac amyloid ATTR and AL, comparing HMDP with DPD and PYP sensitivity and specificity in biopsy-proven cases. Int J Radiol Radiat Ther. 2024;11(5):145-156. DOI: 10.15406/ijrrt.2024.11.00403
Background and purpose: Cardiac amyloidosis (CA) is an underdiagnosed cause of heart failure with preserved ejection fraction. Two types of cardiac amyloidosis, transthyretin (ATTR) and light-chain (AL), are treated differently. Technetium diphosphonate (99mTc-DPD) and pyrophosphate (99mTc-PYP) scintigraphy already have a well-established role in diagnosing ATTR and differentiating it from AL CA. Technetium Hydroxymethylene diphosphonate (99mTc-HMDP) is another radiopharmaceutical that has been studied for the same purpose and acknowledged by the ASNC and EANM guidelines but has yet to establish a clear role in the clinical. To the best of our knowledge, no published study has compared HMDP to DPD or PYP in the same cohort. The study aims to systematically assess and validate the diagnostic sensitivity and specificity of 99mTc-HMDP whole-body planar scintigraphy in biopsy-proven ATTR CA studies of patients compared to DPD and PYP as a meta-analysis.
Method: A comprehensive online literature search using PubMed, Embase, and Medline databases of studies on HMDP, DPD, and PYP imaging diagnostic values in cardiac amyloidosis was conducted in 2021 to identify high-quality studies on cardiac amyloidosis in single-photon imaging and diagnosis based on HMDP, DPD, and PYP radiopharmaceuticals. The relevant identified studies were filtered based on biopsy-proven patients who had undergone bone scintigraphy, including 20 subjects or more; patients' medical history must be free of other myocardiopathies and tracer-related specificities and sensitivities to distinguish ATTR CA from AL. The quality of the studies was assessed using the NIH Quality Assessment Tool for Before-After (Pre-Post) Studies with No Control Group. Mean sensitivity and specificity of nuclear bone scintigraphy in ATTR differentiation from AL amyloidosis was calculated from the selected studies for the three tracers.
Results: Eight selected studies out of 34 were identified on HMDP, DPD, and PYP bone scintigraphy in cardiac amyloidosis, including 868 (intra-extracardiac) biopsy-proven Patients who were included in the meta-analysis provided the following results: the pooled and calculated sensitivity for ATTR of HMDP, DPD, and PYP (98.33%), (99.58%) and (97.44%); and specificity (98.59%), (87.55%) and (93.75%) respectively. The sensitivity of the tracers in diagnosing AL was 5%, 41.3%, and 35%, and specificity was 2%, 12.8%, and 8.3%, respectively.
Conclusion: A meta-analysis of the literature shows Bone tracer HMDP has equivalent sensitivity and higher specificity compared with DPD and PYP for diagnosing ATTR cardiac amyloidosis from AL and can be used with equivalent confidence.
Keywords: HMDP, PYP, DPD, amyloidosis, ATTR, AL, scintigraphy, biopsy, sensitivity, specificity
CA, cardiac amyloidosis; ATTR, transthyretin; AL, light-chain; 99mTc-DPD or DPD, technetium diphosphonate; 99mTc-PYP or PYP, pyrophosphate; HFpEF, heart failure with preserved ejection fraction; PET, positron emission tomography
Systemic amyloidosis is a rare disorder characterised by extracellular deposition or infiltration of myocardium by amyloid protein, leading to loss of normal function and standard organ tissue structure, most commonly heart failure with preserved LV ejection fraction. Cardiac amyloidosis (CA) has a high mortality rate with an average survival rate of 6 months for light-chain (AL) CA and 3 to 5 years for ATTR CA if left undiagnosed and untreated.1 Cardiac involvement is primarily encountered in immunoglobulin light-chain (AL) and transthyretin-associated (hereditary/familial and senile) amyloidosis. The mechanism of phosphate-based radiopharmaceuticals (bone tracers) accumulation in affected myocardium is poorly understood. However, it is hypothesised that the affinity of the tracers in ATTR CA is due to phosphate binding to the high calcium content present in ATTR amyloid fibrils.2
Cardiac amyloidosis diagnosed by invasive endomyocardial or extracardiac tissue biopsy has very high accuracy. Echocardiography of amyloidosis and histological confirmation of amyloid deposition can be involved in non-invasively diagnosing algorithms.3 Early and accurate diagnosis of CA is the key to properly managing the disease progression. It can be treated with emerging novel therapies or chemotherapies depending on the type of amyloidosis. Noninvasive imaging can identify patients with severe and later stages of the disease in a shorter time, increasing the benefit from the available therapy options. Radionuclide bone scintigraphy has the advantage of detecting deposits of cardiac ATTR amyloid protein in the early stages of the disease and distinguishes amyloid of ATTR from AL. By contrast, echocardiography and cardiac magnetic resonance (CMR) can detect abnormal cardiac structural and functional changes due to amyloid deposition in a later stage of the disease, unlike bone scintigraphy.4,5
There has been a history of many bone tracers accumulating in soft tissue, specifically in the heart. Mainly, the two radiopharmaceuticals, DPD and PYP, have been well known to have a clear role in diagnosing CA. HMDP is a relatively more common bone imaging tracer in SPECT due to its availability and reduced cost. Current guidelines have set proper imaging protocol and identified reporting criteria of HMDP scintigraphy for CA. However, HMDP is underutilised for this purpose. Some case studies have been published where incidental cardiac uptake was seen during metastatic workup, follow-up, and other indications with HMDP, but ATTR CA could not be confidently diagnosed.
This study aims to evaluate the published literature, select quality-relevant publications, and pool their results to form a meta-analysis of published results regarding scintigraphic bone tracers in diagnosing ATTR cardiac amyloidosis and whether HMDP may be used with confidence. This review was carried out to look at studies published in PubMed, Embase, and Medline to identify the sensitivity and specificity of HMDP and establish the necessity of obtaining PYP or DPD specifically for cardiac amyloidosis cases if HMDP is already available, which can serve as both, a bone scan agent and CA scan agent, to minimise resources.
The history of CA bone-seeking radiopharmaceuticals detecting amyloid deposition in the heart and other body organs goes back to 1977. EHDP and Tc-PYP scans were performed, and cardiac uptake was then proven by biopsy.6,7 Technetium pyrophosphate (99mTc-PYP) was first formally introduced in the diagnostics of CA in 1983.8 In the last few years, the incidence of CA detection has increased. Endomyocardial biopsy has been the golden standard to confirm the presence of amyloid protein deposition into the tissue and identify the amyloid's subtype. With the increased need for early and noninvasive diagnoses to treat the disease with an appropriate management plan, having HMDP bone scintigraphy radiotracer that is readily available in the nuclear medicine department daily than DPD and PYP, and with the increase of incidental findings of cardiac amyloidosis in skeletal scintigraphy, this study aims to address the question whether HMDP can reveal the diagnosis of CA with high confidence level? Would a nuclear medicine department be able to use DPD, PYP, and HMDP interchangeably? What if DPD or PYP is not a licensed tracer in a department? Would HMDP be a radiopharmaceutical a suitable substitute choice? If a patient has had an HMDP bone scan with incidental findings of cardiac uptake, does a DPD scan have to be done separately to confirm the presence of CA and identify the specific subtype?
Nuclear medicine bone scintigraphy has been tested for positive and negative likelihood ratios in distinguishing ATTR from AL amyloidosis in the myocardium. The diagram (Figure 1)1 shows the difference between the normal myocardium and the LV hypertrophy and increased LV mass due to amyloid deposition in the cardiac wall. Both conditions would show tracer uptake. Therefore, the accumulation of bone tracer is non-specific on its own without the correlation with other clinical tests, including echocardiography and CMR, histology, immunohistochemistry, proteomic analysis, monoclonal protein studies, and genetic testing.3 Figure 2 demonstrates the advantages of combining multimodality imaging to diagnose CA types accurately.9 One study shows that the accumulation of bone tracers is not due to macrophage infiltration; it was found to be significantly lower in ATTR amyloid than in AL amyloid. However, increased microcalcifications in ATTR amyloid may explain why bone tracers accumulate more in ATTR than AL. Also, microcalcifications densities were found in some AL cases, which might be the reason for positive bone scintigraphy in those cases.10 Other possibilities of cardiac uptake in ATTR CA may be due to different compositions of amyloid fibrils, different affinities of the bone tracer for amyloid proteins, differences in tissue involvement, or differences in their chemical structure diagram.1,5 Deposited proteins in the myocardium could be either Transthyretin proteins originating from the liver or immunoglobulin light-chain proteins derived from a clone of plasma cells (Figure 2).5 The exact mechanism of bone-seeking tracers binding to amyloid is still unclear.11 Thus, some common bone tracers have yet to be validated for this purpose.
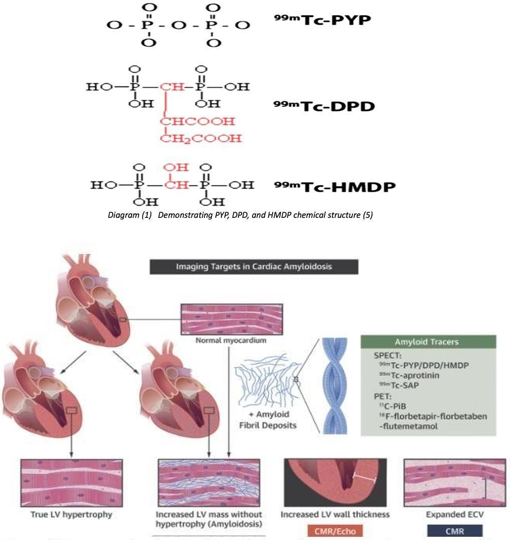
Figure 1 Demonstrating increased wall thickness due to hypertrophy versus amyloid deposition into the wall leading to abnormality of the wall.
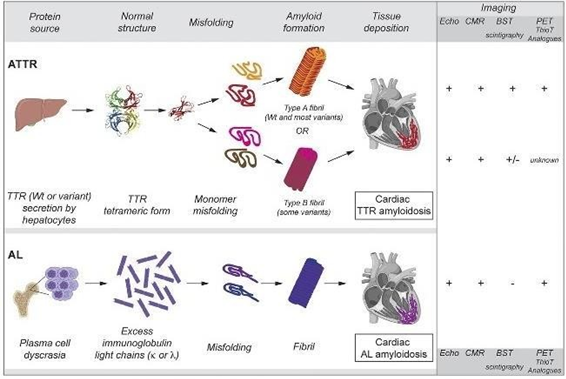
Figure 2 Demonstrating The origin of a specific type of amyloid protein and the shape of misfolding, and role of multi modalities imaging in detection of each type of the cardiac amyloidosis present in myocardium.9
Method (Search strategies and data collection)
This meta-analysis complied with the Preferred Reporting Items for Systematic Reviews and Meta-Analysis of Diagnostic Test Accuracy Studies guidelines.12
The author conducted a comprehensive online literature search in Medline, Embase, and PubMed databases of studies on HMDP, DPD, and PYP imaging diagnostic role in cardiac amyloidosis to identify studies with sensitivities and specificities of the tracers in the targeted population. Initially, no exclusion criteria were applied. A systematic search was done in Medline and Embase databases through Ovid (ovidsp.ovid.com) using the following strategies, and a pooled number of citations are identified for each database per question in the table below (Table 1):
|
Searching Question |
Embase |
Medline |
|
Exp heart amyloidosis or cardiac amyloid.mp. or cardiac amyloidosis.mp. or cardiac anyloidoses.mp. or exp ATTR or exp AL AND exp oxidronate technetium tc 99m or HDP.mp. or hydroxymethylene.mp. or HMDP.mp. or 99mTc- HMDP.mp. AND exp bone scintiscanning or bone scintiscanning.mp. or scan.mp. or imaging.mp. or exp scintigraphy or scintigraphy.mp. or bone scan.mp |
130 |
35 |
|
Exp heart amyloidosis/ or cardiac amyloid.mp. or cardiac amyloidosis.mp. or cardiac anyloidoses.mp. or exp ATTR/ or exp AL/ AND DPD.mp. or exp Diphosphonates/ or propanodicarboxylicacid.mp. or exp Radionuclide Imaging/ or 99mTc-DPD.mp. AND exp bone scintiscanning or bone scintiscanning.mp. or scan.mp. or imaging.mp. or exp scintigraphy or scintigraphy.mp. or bone scan.mp. |
1036 |
295 |
|
Exp heart amyloidosis/ or cardiac amyloid.mp. or cardiac amyloidosis.mp. or cardiac anyloidoses.mp. or exp ATTR/ or exp AL/ AND exp pyrophosphate technetium tc 99m/ or PYP.mp. or exp pyrophosphate/ or Pyrophosphate.mp. or 99m Tc-PYP.mp. or 99mTc Pyrophosphate.mp. AND exp bone scintiscanning or bone scintiscanning.mp. or scan.mp. or imaging.mp. or exp scintigraphy or scintigraphy.mp. or bone scan.mp. |
437 |
119 |
Table 1 The search strategy in Medline and Embase, shows the identified citations
Also, another systematic search was done in the PubMed database using the following strategy, and a pooled number of citations were identified (Table 2):
|
Searching Question |
PubMed Citation |
|
((cardiac amyloid or cardiac amyloidosis or heart amyloid or heart amyloidosis or cardiac amyloidoses or ATTR or transthyretin or AL or light-chain) AND (diagnostic or imaging or SPECT or SPET or images or scan or scintigraphy or diagnosis)) AND (HDP or HMDP or PYP or DPD or Diphosphonate or hydroxymethylene or Tc or Technetium) |
1650 |
|
((cardiac amyloid or cardiac amyloidosis or cardiac amyloidoses) AND (HDP or hydroxymethylene or HMDP or 99mTc-HMDP)) AND (scan or imaging or scintigraphy or bone scan) |
49 |
|
((cardiac amyloid or cardiac amyloidosis or cardiac amyloidoses) AND (DPD or propanodicarboxylicacid or 99mTc-DPD)) AND (scan or imaging or scintigraphy or bone scan) |
90 |
|
((cardiac amyloid or cardiac amyloidosis or cardiac amyloidoses) AND (PYP or Pyrophosphate or 99m Tc- PYP or 99mTc Pyrophosphate)) AND (scan or imaging or scintigraphy or bone scan) |
158 |
|
((cardiac amyloid or cardiac amyloidosis or cardiac amyloidoses) AND (HDP or hydroxymethylene or HMDP or 99mTc-HMDP or DPD or propanodicarboxylicacid or 99mTc-DPD or PYP or Pyrophosphate or 99m Tc-PYP or 99mTc Pyrophosphate)) AND (scan or imaging or scintigraphy or bone scan) |
272 |
|
(((cardiac amyloid or cardiac amyloidosis or heart amyloid or heart amyloidosis or cardiac amyloidoses) AND (ATTR or transthyretin or TTR or AL or light-chain)) AND (HDP or HMDP or PYP or DPD or Diphosphonate or hydroxymethylene or 99mTc Pyrophosphate or Tc or Technetium)) AND (diagnostic or diagnosis or imaging or images or radionuclide or SPECT or SPET or scan or scintigraphy) |
245 |
Table 2 Searching strategy in PubMed showing identified citation
Further relevant publications were found via manual inspection of the retrieved resources.
Seven additional publications were identified. To search as comprehensively as possible, including "grey literature" on HMDP’s role in cardiac amyloidosis was searched. No relevant studies were found. No language restrictions were applied to the search. Search results were screened independently after duplicates were removed using the automated tool in Zotero. The initial search was completed on July 20th and again updated on July 30th, 2021. First, Study titles and abstracts were screened. Then, full texts of possibly eligible studies were retrieved for review.
Inclusion and exclusion criteria
Studies were assessed for the following criteria: biopsy-proven CA patients going under HMDP, DPD, or PYP bone scintigraphy. Sample size ³ 20 was chosen to increase the accuracy of defined sensitivity and specificity in diagnosing and distinguishing ATTR CA from AL. Also, studies with defined sensitivity and specificity (7 studies) and studies provided sufficient data to determine sensitivity and specificity (1 study). Studies were excluded for the following reasons: suspected patients presented with other cardiac pathologies, abstracts only or conference abstracts, case studies, systematic reviews and meta-analysis, duplicate reports, letters to the editor, PET and PET/CT studies, 99mTc-MDP studies, irrelevant publications, and insufficient data for determining sensitivity and specificity. Of studies with a large cohort, only the number of biopsy-proven patients were selected for our analysis.
Data extraction and Quality assessment
The data were independently extracted in an Excel sheet by the author. The following data were extracted from the selected studies: study sample size, total patients who underwent bone tracer scintigraphy with suspected CA diagnosis, tracer specified for each study, uptake assessment methodology, biopsy-proven subjects with final diagnoses with ATTR and AL cardiac amyloidosis, patient demographic information (age and gender), sensitivity, specificity, true positive, false negative and false positive.
Even though few studies have used a quantitative method for calculating the ratio of cardiac uptake to ribs, the Perugini method was dominantly used to identify the degree of cardiac uptake (scoring system, 0-3) in all the selected studies. Notably, subjects in various studies with a score of 1 have been considered positive and negative in others due to no clear cut that could be identified. Therefore, our analysis considered subjects with Perugini Grade ≥ 1 positive and Perugini Grade <1 negative.
Eligible studies were assessed for quality using the NIH Quality Assessment Tool for Before-After (Pre-Post) Studies with No Control Group. The quality assessment tool consists of 12 questions, and the overall quality of the studies was good and fair.
Statistical analysis
Pre-specified inclusion criteria, including proven biopsy as a gold standard, led to the selection of a minimal number of studies per tracer. The meta-analysis included only three studies on the tracer of interest (HMDP) with a cumulative sample size of (n=204). A similar number of studies for DPD and PYP were selected to compare the sensitivity and specificity in a comparable sample size. Therefore, the sensitivity and specificity of these carefully selected high-quality biopsy-proven studies are presented without further statistical analysis.
All the papers selected in our meta-analysis have conformed to the standardised protocol for each of the three tracers.
Standard DPD and HMDP protocol scintigraphy by ASNC and EANM
The American Society of Nuclear Cardiology and the European Association of Nuclear Medicine have published guidelines and protocols,13 and Perugini published reporting guidelines.14 (Table 3)
|
Camera |
Anger gamma camera |
|
Preparation |
No specific test preparation is required |
|
Radiopharmaceutical |
99mTc-DPD or HMDP |
|
Dose range |
10–20 mCi (370-740 MBq) |
|
Average dose |
700-740MBq |
|
Imaging mode |
Recommended: Chest Planar or Chest/Cardiac SPECT Required: Cardiac/Chest SPECT if planar is positive Optional: Whole-body planar imaging |
|
Scan time |
two to three hours after intravenous injection of 99mTc-DPD or HMDP |
|
Position |
Supine |
|
Matrix |
Planar: 256 by 256, at least 64 by 64 is required SPECT:128 by 128, at least 64 by 64 is required |
|
Image duration |
Count based: 750,000 counts or 20 cm per minute |
|
Energy window |
140 keV, 15–20% |
|
Collimators |
Low energy, high resolution |
|
SPECT mode |
Number of views/detector (40 views / detector) Time per view (30 seconds per view) Magnification (1.0) |
|
Reporting |
Semiquantitative (Perugini): Cardiac uptake of 99mTc-DPD and HMDP visual comparison to bone (rib) uptake at 3 hours |
|
Reporting guidelines |
Score 0, absent cardiac uptake and normal bone uptake; Score 1, mild cardiac uptake, inferior to bone uptake Score 2, moderate cardiac uptake accompanied by attenuated bone uptake Score 3, strong cardiac uptake with mild/absent bone uptake |
Table 3 Slandered ASNC and EANM Protocol for HMDP and DPD cardiac amyloidosis scintigraphy
The comprehensive online search of Medline, Embase and PubMed resulted in 4550 articles. An additional seven studies were identified from other references, and relevant studies were found in the references to other articles. Of the 4557 reports, (2725) duplicates were removed by the automated tool. Pre-screening the complete text, by abstracts and titles, irrelevant and PET studies, 1317 and 49, respectively, were excluded. The remaining (466) studies were screened, not in the field of interest or insufficient data (348), case studies (45), meta-analysis (3), and abstract only (17) were also excluded. Fifty-three reports were set for retrieval, of which 19 reports were not retrieved. Thus, 33 articles were selected and retrieved in full-text version.15-46 Twenty-five studies were ineligible for the following reasons: letters to editors, protocols, non-biopsy patient studies, studies biopsy-proven patient < n = 20, abstract only, and sensitivities or specificities were not defined. The eligible eight studies including 731 patients assessing the sensitivity and specificity with the reference of biopsy for HMDP,15,20,24 and DPD,20,30,33,34 and PYP20,37,41 meeting the inclusion criteria were included in this meta-analysis. The following diagram2 illustrates the exclusion and inclusion process of the studies.
From: Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ 2021;372:n71. doi: 10.1136/bmj.n71 For more information, visit: http://www.prisma-statement.org/1
Patient population
Studies were selected based on the pre-specified inclusion criteria. The biopsy-proven patients' data were extracted from the eligible studies, including 868 patients were divided into four categories: ATTR biopsy positive, AL biopsy Positive, biopsy negative for amyloid deposition, but the bone scintigraphy was positive, and negative bone scintigraphy corresponding with biopsy negative for amyloid deposition. The subgroups for ATTR biopsy positive are ATTR biopsy positive and scan positive, ATTR biopsy positive with negative scan, and AL biopsy positive subgroup is biopsy and scan positive for AL. Also, these groups were classified as false positive scans when CA biopsy was negative or false positive for ATTR with AL positive biopsy and scintigraphy. Subjects in subgroups biopsy negative and scan negative for ATTR and AL were true negative and served as the control group; however, zeros indicate no control group was involved in the study. Table 5,6 summarises the number of the classified subjects in each subcategory. (Figure 3) (Table 4)
|
|
Male |
Female |
Undefined |
|
HMDP (n=147) |
94 |
40 |
13 |
|
PYP (n=229) |
105 |
43 |
81 |
|
DPD (n=360) |
122 |
29 |
209 |
Table 4 Demonstrating number of male patients versus female patients in underwent bone scintigraphy with different tracers. The pie chart shows the percentage of men across all studies compare to woman. The unknown portion of that chart area is of unreported patients' gender
|
CA (Arift+AL) Biopsy Proven Patients |
|
|
HDP |
147 |
|
PYP |
229 |
|
DPD |
360 |
Table 5 Demonstrating only biopsy proven patient who underwent bone scan for each tracer
|
Study |
CA (AM+ AL) Biopsy proven Positive Or Negative |
ATTR Biopsy positive |
Biopsy positive Scan positive for ATTR (True Positive) |
ATTR Biopsy positive Scan negative (False negative) |
AL Biopsy positive |
Biopsy positive Scan positive For AL |
Biopsy negative Scan positive (False positive) |
Biopsy negative Scan negative (True negative) |
|
Cappelli15 |
85 |
39 |
39 |
0 |
26 |
2 |
0 |
20 |
|
Gillmore20 |
21 |
14 |
14 |
0 |
4 |
0 |
0 |
3 |
|
Gala24 |
98 |
47 |
45 |
2 |
14 |
1 |
0 |
37 |
|
Hutt30 |
51 |
37 |
37 |
0 |
14 |
8 |
0 |
0 |
|
Rapezze33 |
94 |
45 |
45 |
0 |
34 |
11 |
0 |
15 |
|
Gillmore4 |
240 |
162 |
161 |
1 |
43 |
22 |
4 |
31 |
|
Moore34 |
21 |
13 |
13 |
0 |
8 |
2 |
0 |
0 |
|
Bokhari37 |
45 |
33 |
32 |
1 |
12 |
4 |
0 |
0 |
|
Poterucha41 |
104 |
69 |
68 |
1 |
13 |
5 |
0 |
22 |
|
Gillmore4 |
109 |
85 |
84 |
1 |
15 |
5 |
2 |
7 |
|
Total |
868 |
544 |
538 |
6 |
183 |
60 |
6 |
135 |
|
|
(100%) |
(62.6%) |
|
|
(21.1%) |
|
(0.69%) |
(15.6%) |
Table 6 Demonstrating data of only biopsy proven patient either positive or negative, and whether the bone scintigraphy was positive for the amyloid specific type.
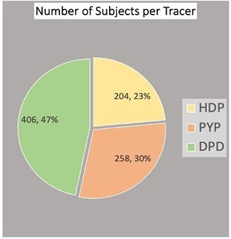
Figure 3 Demonstrating number of patient participated in different studies, 406 patient underwent DPD bone scintigraphy, 258 patients underwent PYP bone scintigraphy, and 204 patient underwent HMDP scintigraphy.
The eight selected studies with pre‐specified criteria for diagnosing CA are summarised in table 7 and displayed graphically in Figure 4-6. Figure 5 displays the results of ATTR: 99mTc-HMDP has the mean sensitivity and specificity for ATTR of 98.33% and 98.12%, respectively, compared to DPD and PYP showed mean sensitivity for ATTR CA of 99.58% and 96.33%, and specificity of 87.55%, 91.57%, respectively. HMDP performed very well (outperforming specificity) compared to the standard DPD and PYP tracers.
|
Study |
Tracer |
Sensitivity for amyloid |
Specificity for amyloid |
Sensitivity for ATTR |
Specificity for ATTR |
Sensitivity for AL |
Specificity for AL |
Prevalence |
|
Cappelli15 |
HMDP |
61.90% |
100.00% |
100.00% |
96.00% |
7.69% |
4.00% |
76.47% |
|
Gillmore20 |
HMDP |
77.00% |
100.00% |
100.00% |
100.00% |
0.00% |
0.00% |
85.70% |
|
Gala24 |
HMDP |
77.50% |
100.00% |
95.00% |
98.36% |
7.14% |
2.00% |
62.20% |
|
Hutt30 |
DPD |
86.00% |
100.00% |
98.30% |
89.30% |
51.10% |
10.70% |
100.00% |
|
Rapezze33 |
DPD |
66.10% |
100.00% |
100.00% |
84.30% |
57.10% |
15.70% |
84.04% |
|
Gillmore4 |
DPD |
88.50% |
88.50% |
100.00% |
86.10% |
32.00% |
13.90% |
85.40% |
|
Moore34 |
DPD |
68.40% |
100.00% |
100.00% |
90.50% |
25.00% |
9.50% |
100.00% |
|
Bokhari37 |
PYP |
80.40% |
100.00% |
98.00% |
95.00% |
33.30% |
5.00% |
100.00% |
|
Poterucha41 |
PYP |
89.60% |
100.00% |
97.00% |
91.00% |
33.30% |
9.00% |
78.85% |
|
Gillmore4 |
PYP |
98.47% |
77.80% |
94.00% |
89.00% |
38.00% |
11.00% |
91.70% |
Table 7 Demonstrating Bone scintigraphy for diagnosis of ATTR outcomes with the reference test of biopsy, sensitivity and specificity for amyloid in general is demonstrated in column 2 and 3, sensitivity and specificity for ATTR is demonstrated in column 4 and 5, sensitivity and specificity for AL
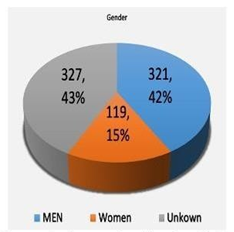
Figure 4 Demonstrating number of male patients versus female patients in underwent bone scintigraphy with different tracers. The pie chart shows the percentage of men across all studies compare to woman. The unknown portion of that chart area is of unreported patients' gender.
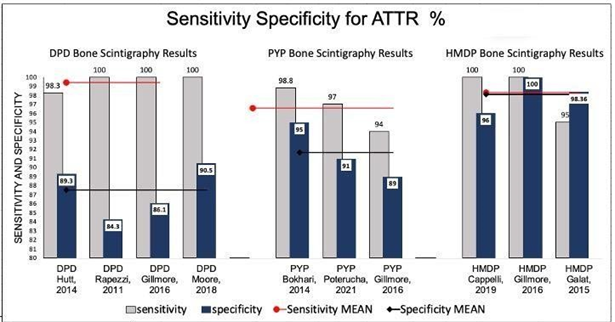
Figure 5 Demonstrating sensitivities and specificities of each study for the selected tracer that was utilized for bone scintigraphy for diagnosis of ATTR CA, mean sensitivity and specificity of each trace related studies are shown across the studies figures. Comparison of sensitivities and specificities is made for the tracers of interest.
Pooling and assessing data across all eight studies with the three tracers (99mTc‐HMDP, 99mTc‐DPD, or 99mTc‐PYP) evaluated their role in differentiating ATTR from AL despite high sensitivities and specificities for ATTR. Figure 6 displays the results for AL: 99mTc—HMDP has the mean sensitivity and specificity for AL of 4.94% and 2%, respectively, compared to DPD and PYP, which showed mean sensitivity for AL CA of 41.30% and 34.87% and specificity of 12.75% and 8.33%, respectively. (Figure 6) (Table 7)
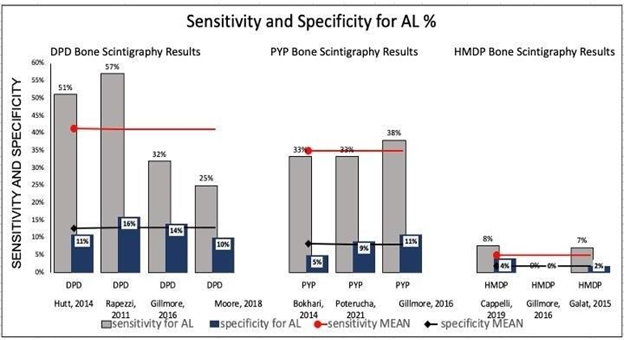
Figure 6 Demonstrating sensitivities and specificities for AL CA of each study for the selected tracer that was utilized for bone scintigraphy for diagnosis of ATTR CA, mean sensitivity and specificity of each trace related studies are shown across the studies figures. Comparison of sensitivities and specificities is made for the tracers of interest.
1PRISMA 2020 flow diagram for new systematic reviews, which included searches of databases and registers only
The first case of a patient suspected of CA with a kidney transplant was referred to our nuclear medicine department at Guy's and St Thomas' Hospital. The DPD whole-body planar imaging shows normal bone uptake and absent cardiac uptake. (Figure 7)
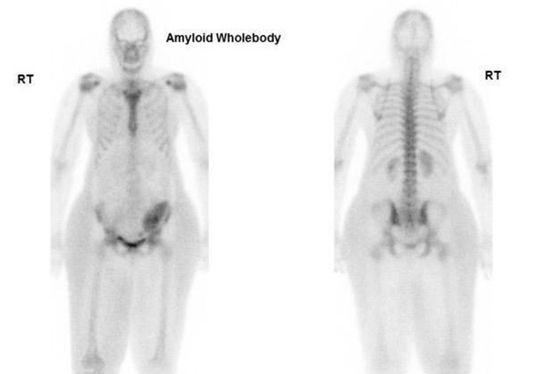
Figure 7 Demonstrating DPD bone scan of a patient undergoing cardiac amyloid study showing no cardiac uptake and normal bone uptake. Kindly contributed by Dr Charlotte Fowler, Guy's and St Thomas' Hospital.
The second case involved a suspected CA patient who was referred to our nuclear medicine department at Guy's and St Thomas' Hospital. The DPD whole-body planar imaging shows defused and attenuated bone uptake and Perugini Grade 2 cardiac uptake. (Figure 8)
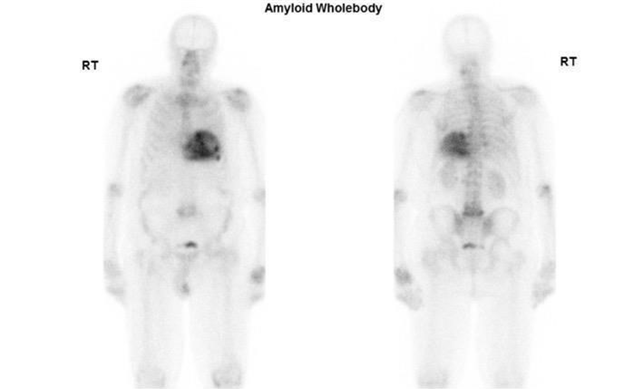
Figure 8 Demonstrating DPD bone scan of a patient undergoing cardiac amyloid study showing intense cardiac uptake. Perugini Grade 2. Kindly contributed by Dr Hajira /lyas, Guy's and St Thomas' Hospital.
This is an online published case report of a patient who underwent an MDP bone scan for metastatic workup. The scan was negative for the mother’s reason. However, mild cardiac uptake was incidentally detected. Later, a PYP scan was performed to confirm the diagnosis of CA, which resulted in intense cardiac uptake with the final diagnosis of ATTR CA. (Figure 9)
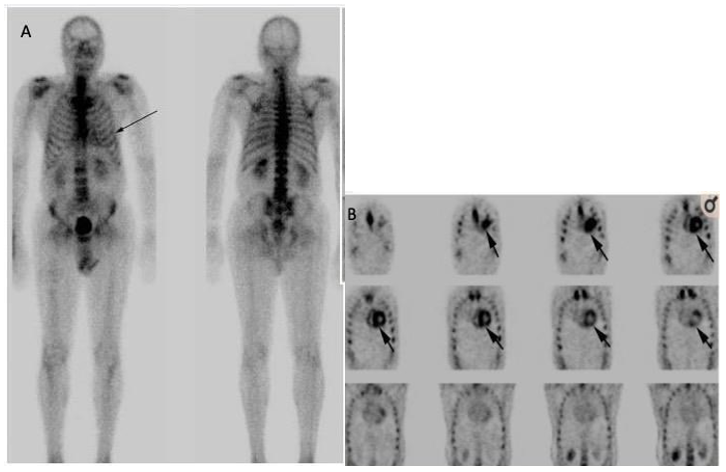
Figure 9 Demonstrating MDP bone scan of a patient undergoing metastatic work up, which was negative metastatic disease but raised suspicion of CA due to mild uptake in the cardiac, PYP was done after MDP bone scan to confirmed the diagnosis of CA. Published by Fathala, 202051
(A) MDP bone scan for metastatic work up, showing normal bone and mild uptake in cardiac.
(B) PYP bone scan for suspected cardiac amyloidosis, showing normal bone and intense cardiac uptake.
Nuclear medicine bone scintigraphy is one of the most frequent tests done in nuclear medicine utilising different tracers. The incidence of cardiac amyloidosis has increased for the last few years, and the need to diagnose CA non-invasively has increased. 99mTc-DPD and 99mTC-PYP have a well-established role in diagnosing CA, whereas 99mTc-HMDP is a more widely available tracer, and there has published guidelines for DPD and HMPD by EANM.36 However, it is underutilised for the diagnosis of ATTR- CA. Many studies have noted that MDP bone scintigraphy is not recommended for this purpose due to its low sensitivity to CA. Thus, HMDP is the tracer of interest in this study.
We conducted a systematic review and meta-analysis of eight studies on DPD, PYP, and HMDP bone scintigraphy to diagnose ATTR-CA findings in 868 biopsy-proven patients. Despite the small number of biopsy-proven patients evaluated by the HMDP tracer, we have found that it is susceptible and specific for detecting CA and differentiating ATTR from AL. Having tissue biopsy as a gold standard reference test for cardiac amyloidosis diagnosis, positive HMDP bone scintigraphy for cardiac uptake indicates the presence of the disease to a very great extent. Our analysis considered patients with visual scores of 1-3 of cardiac uptake as positive and any defused or absent tracer uptake in the myocardium was considered a negative scan. The pooled DPD and PYP sensitivities for ATTR CA of 99.58%, 96.33%, and specificity of 87.55% and 91.67%, respectively, were compared to HMDP sensitivity for ATTR CA of 98.33% and specificity of 98.12%.
The results of our meta-analysis indicate that HMDP can be used interchangeably with DPD and PYP with equivalent confidence. The presence of DPD, PYP, or HMDP myocardial uptake (grade 1, 2, or 3) was found to have a sensitivity and specificity for diagnosing ATTR CA. False-positive scans with biopsy negative or biopsy positive for AL CA reduce the specificity of any tracer of interest. Therefore, the noninvasive HMDP bone scintigraphy diagnostic tool of CA should be combined with serum/urine testing for the detectable clonal immunoglobulins to exclude AL cardiac amyloidosis.
Other recently published case studies demonstrate the sensitivity and specificity of HMDP bone scintigraphy. A recent case report shows HMDP was used to confirm the diagnosis of ATTR after an MRI showed abnormal anatomy of the cardiac wall with diffused asymmetric hypertrophy of the right and LV walls, and endomyocardial biopsy was positive for ATTR amyloidosis.47
This study confirms the diagnostic utility of HMDP in this clinical context. Notably, our results are applicable to suspected CA patients having no other cardiac pathologies. Since most of the current studies address only those patients who have been referred from cardiology with suspicion of cardiac amyloid, this study cannot be used to establish sensitivity and specificity of incidentally identified cardiac uptake in patients undergoing HMDP scans for other reasons. Those patients undergoing bone scintigraphy for other indications will have a lower prevalence, and the sensitivity and specificity depend on the prevalence of any condition. In a larger population, the sensitivity may remain high. However, the specificity could be lower due to other conditions of LV hypertrophy that can show an accumulation of bone tracers in the heart (e.g., myocardial ischemia, metabolic disorders with hypercalcemia, infiltrative diseases such as sarcoidosis, or inflammatory myocarditis).48 Another meta-analysis has displayed similar results of bone-seeking tracers. The study concluded that bone scintigraphy is a practical tool in diagnosing cardiac amyloidosis.49
As PYP and DPD scans have suggested that they are able to distinguish ATTR from AL, our results show that HMDP follows the same concept. The advantages of identifying HMDP's ability to detect CA and differentiate ATTR from AL can help us to recognise the early pathophysiological process of cardiac amyloidosis with the potential for monitoring treatment response without repeating another scintigraphy with different tracer, which reduces further radiation exposure to the patients.
Incidental case reports
Since HMDP is one of the most frequent tracers used for bone scintigraphy, there is a high possibility of cardiac uptake appearing in random patients going under bone scintigraphy for other indications (e.g., prostate cancer workup), especially in male patients over 60. Many cases of incidental findings on HMDP bone scans were published. For imaging results correlation and comparison, the degree of uptake in the cardiac and bone tracer accumulation (Perugini scoring system—Figure 10)23 is correlated with disease severity.50
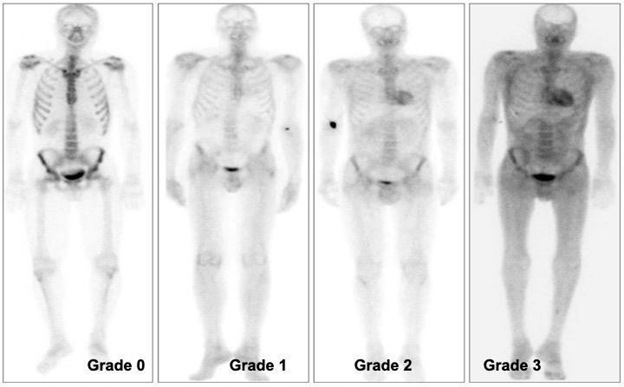
Figure 10 Demonstrating visual scoring of 0- 3. (0) absent cardiac uptake and normal bone uptake, (1) mild cardiac uptake, inferior to bone uptake, (2) moderate cardiac uptake associated with attenuated bone uptake, and (3) high cardiac uptake with decreased or absent bone uptake. Published by Glaudemans, 2014.23
HMDP
We present a case report from our Nuclear Medicine department at Guy's and St Thomas' NHS Foundation Trust. A patient presented with a known history of malignant neoplasm of the prostate, type 2 diabetes, hyperlipidemia NOS, chronic kidney disease, and no history of cardiac myopathies. The patient was scheduled for an HMDP bone scan as a prostate cancer patient complaining of hip bone pain. The scan was carried out as normal bone scintigraphy with three hours post-injection delayed phase of whole-body imaging. While reviewing and reporting the whole-body images, a note was made of the clear, intense uptake in the heart visually assed of a Perugini grade 2. The findings were highly associated with amyloidosis (Figure 11). A recommendation for a referral to the cardiology was made for further investigation. However, no further investigation was done at six-week intervals by the time of publication.
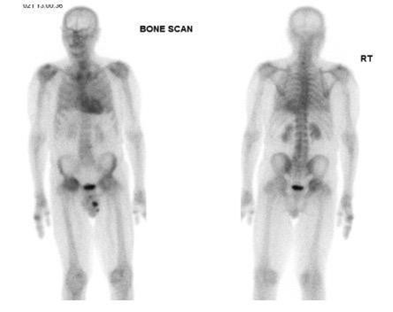
Figure 11 Demonstrating HMDP bone scan of a patient undergoing cardiac amyloid study showing Perugini Grade 2 cardiac uptake. Kindly contributed by Dr Dhruba Dasgupta, Guy's and St Thomas' Hospital.
The following published case of ATTR CA diagnosed due to incidental myocardial uptake during bone scintigraphy is similar to our case from Guy's and St Thomas' NHS.
Foundation Trust. In this case, an 83-year-old man underwent prostatectomy for prostate cancer with a known history of carpal tunnel syndrome. The patient underwent serial (99mTc-HMDP) scintigraphy to evaluate bone metastasis due to prostate cancer. Incidental finding of cardiac uptake (visual grade, 2; heart-to-contralateral (H/CL) ratio, 1.70) was observed. However, no further investigation was made on suspected ATTR since the patient had no symptoms of heart failure. 99mTc-HMDP Bone scan was repeated Six years later for re-elevation of prostate-specific antigen level. The cardiac uptake was found to be greater (visual grade 3, and H/CL ratio: 2.32), echocardiography showed the left ventricular thickness, and endomyocardial biopsy showed amyloid deposition in the cardiac. The presence of ATTR was confirmed by immunohistochemistry.51
A further report of a 76-year-old man with metastatic prostate cancer who underwent 99mTc-HMDP bone scintigraphy found an incidental diffuse left ventricular abnormal uptake suggesting ATTR cardiac amyloidosis. The patient also underwent an 18-F NaF PET/CT bone scan, but no cardiac uptake was noted in the 18-F NaF PET/CT imaging.2
When cardiac uptake was detected in the MDP bone scan, a PYP bone scan was done to confirm the CA's presence and differentiate ATTR from AL CA. The presented case of HMDP (Guy's and St Thomas' Hospital). A patient with no history of cardiac pathologies presented with the question of prostate recurrence (see clinical history in study case 1), regarding incidental findings, the cardiac tracer uptake strongly correlated with suggestive ATTR positive; however, further clinical evaluation of incidental cardiac uptake on bone scintigraphy is warranted.52,53
Other radiopharmaceuticals MDP
Other traces of bone scintigraphy are still under investigation and have very low sensitivity for CA. 99m TC-MDP (99mTc-methylene diphosphonate) is also a very common bone scan tracer. However, the reported sensitivity of the tracer is very low.54 Other studies stated that The tracers 99mTc-MDP and 99mTc-aprotinin are not recommended for this purpose.55
The last case we present here is a recently published for incidentally detected cardiac amyloidosis on 99mTc-MDP bone scintigraphy. An 86-year-old man with prostate cancer treated several years ago presented with elevated prostate-specific antigen and back pain with a known history of hypertension, hyperlipidemia, and heart failure with preserved ejection fraction (HFpEF). A 99mTc-MDP scan was indicated to rule out bone metastasis. The scan findings were negative for metastatic disease. However, degenerative changes in multiple joints were noted, and the tracer's unexpected mild, diffuse myocardial uptake was further investigated by performing 99mTc-PYP for suspected CA. The PYP scintigraphy demonstrated intense myocardial uptake in both planar and SPECT. Histologically, by Serum immunofixation, AL was ruled out, confirming the uptake was consistent with ATTR CA. The patient was referred to the cardiology department for further treatment (Figure 9).56 In another small cohort study of 19 biopsy-proven patients who underwent DPD and MDP scans. In whom MDP showed a mild cardiac uptake 0-1by the Perugini method, DPD demonstrated positive for the patients diagnosed with ATTR.54 (Figure 12)
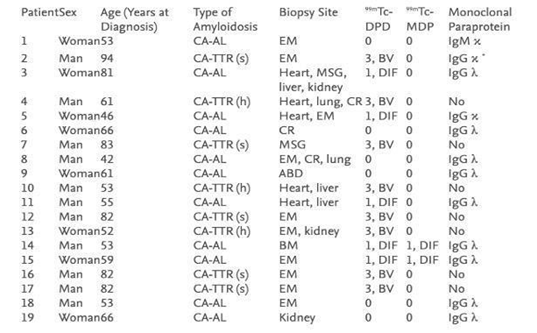
Figure 12 Demonstrating results of DPD and MDP bone scan in 19 patients undergoing cardiac amyloid study showing no cardiac uptake in MDP where as DPD is positive in most cases. Published by F. Javier, 2012.53
ABD, abdomen-peritoneum; BM, bone marrow; By, biventricular; CA-AL, light chain cardiac amyloidosis; CA-TTR: transthyretin-related cardiac amyloidosis (h: hereditary; s: senile); CR, colorectum; DIF, diffuse; EM, endomyocardium; Heart, explanted heart; MSG, minor salivary gland.
Lastly, not to be confused with 123I-labeled serum amyloid P imaging is performed for suspected systemic AL amyloidosis but not cardiac amyloidosis.1
PET tracers
On the other hand, Positron Emission Tomography (PET) has a well-established role in detecting amyloid deposition in many body organs, including the brain. Many tracers, including 18F- NaF and 18F-florbetaben, are limited in diagnosing cardiac amyloidosis. Few studies concluded that differentiating CA subtypes is practicable and might be useful with 18F-florbetaben, but 18F—NaF is significantly inferior to single-photon imaging tracers.50,58,59 Other amyloid-binding PET tracers, C-11–PiB, F-18—florbetapir, and F-18–flutemetamol, have been successful in imaging ATTR and AL cardiac amyloidosis.
This comprehensive database search did not identify studies directly comparing HMDP and PYP or HMDP and DPD in the same cohort of patients. Finally, the general population's sensitivities and specificities differ from incidental findings, which have not been published in much literature (knowledge gap).
There is a need to have a study comparing incidental findings in HMDP bone scans with myocardial left ventricle hypertrophy. Second, Figure11 shows an example of such patients undergoing oncological bone scintigraphy, and with incidental findings, they were recommended for cardiac workup. Occasionally, the referrers neglected cardiac uptake, raising suspicion of CA due to more common cardiac uptakes for other reasons, such as LV infractions. Further work is required to establish the significance of incidental cardiac uptake on HMDP. Therefore, further work and education of oncologists, urologists, and orthopaedic surgeons as frequent bone scan referrers and cardiologists are recommended.
We conducted a systematic review and meta-analysis of eight studies on DPD, PYP, and HMDP bone scintigraphy to diagnose ATTR-CA findings in 868 biopsy-proven patients. Despite the small number of biopsy-proven patients evaluated by HMDP tracer, we have found that it is highly sensitive and specific for detecting CA and differentiating ATTR from AL. We concluded that HMDP is decent for use and equivalent to current guidelines that mandate the role of DPD or PYP. HMDP CA positive bone scan with myocardium uptake can be reported as ATTR CA with the absence of monoclonal protein without performing any further invasive procedures, especially in subjects in whom EMB is unethical.
I wish to extend my special appreciation to Dr. Charlotte Fowler. Many thanks to Dr. Dhruba Dasgupta and Dr. Hajira Ilyas at Guy's and St Thomas' Hospital for contributing study cases.

©2024 Aldehlaui, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.