International Journal of
eISSN: 2574-8084


Clinical Images Volume 5 Issue 6
Department of Radiology, Bichat Hospital, France
Correspondence: Monique Boukobza, Department of Radiology, Assistance Publique-Hopitaux de Paris, Bichat Hospital, 75018, France, Tel + 33 6 24 65 29 32, Fax + 33 1 40 25 83 05
Received: February 13, 2018 | Published: November 19, 2018
Citation: Boukobza M, Laissy JP. The many faces of the reversible cerebral vasoconstriction syndrome (RCVS). Int J Radiol Radiat Ther. 2018;5(6):330-334. DOI: 10.15406/ijrrt.2018.05.00188
The reversible cerebral vasoconstriction syndrome (RVCS), a recently recognized syndrome, is a common cause of sudden headache and of recurrent thunderclap headache (TCH).1A recently recognized syndrome characterized by recurrent TCH attacks. Many reports of this condition began accumulating these last ten years. This syndrome has also been called: Call-Flemming syndrome, benign cerebral angiopathy, post-partum angiopathy. These terms has been unified as RVCS.This name of RVCS was first proposed by Calabrese et al.2in 2007, and these authors define the diagnostic criteria of this clinical-radiological syndrome.2 (i) multifocal segmental cerebral artery vasoconstriction documented by angiographic imaging tests; (ii) no evidence of aneury small subarachnoid hemorrhage; (iii) normal cerebrospinal fluid analysis; (iv) severe and acute headaches with or without additional neurologic signs or symptoms; and (v) reversibility of angiographic abnormalities within 12 weeks after onset.
The laboratory findings have received some recent modifications. Cerebrospinal fluid (CSF) may be normal or near-normal (protein concentration<100 mg/dL,<15 white blood cells/μL, normal glucose).3There is a characteristic pattern of radiographic features alongside suggestive clinical manifestations, which lead to a diagnosis of RVCS.This syndrome is characterized:1-clinically by acute-onset and very severe headache, often thunderclap headache,ie headache peaking in one minute, and with or without acute neurological signs and symptoms, such as seizures, hemiparesis or encephalopathy. 2-radio logically by multifocal and segmental narrowing of multiple cerebral arteries corresponding to vasoconstriction that can affect the proximal, middle, and distal arterial segments and posterior predominant brain edema. The vasoconstriction is demonstrated by a characteristic diffuse "beaded" appearance identified onmagnetic resonance angiography (MRA), angio-CT(ACT) or catheter cerebral angiography.4,5This syndrome is also defined by the complete resolution of the vasoconstriction shown by follow-up on MRA, CTA or catheter cerebral angiography within 12 weeks after onset, together with the total clinical normalization. Epidemiology RVCS affects patients of all ages, with a middle-aged female preponderance, between 20 and 50; the sex ratio varies among the series.The RVCS seems rare and perhaps under-recognized in children.6 Causes: There are various precipitants of RCVS including, sexual activity, exertion, pregnancy-related conditions (early puerperium, pre-eclampsia, and eclampsia; post-partum), sympathomimetic or vasoactive substances, including nasal decongestants, serotonine-reuptake inhibitors, catecholamine-secreting tumors and ergolinederivates, intravenous immunoglobulin, blood products, immune suppressants, and the use of illicit drugs(cannabis, cocaine) or comorbid auto-immune disorders. Many other putative precipitants have been reported. In 2/3 of patients, one or multiple precipitating factors are identified.
In clinical practice, non-enhanced CT scan is the initial performed examination. When convexal subarachnoid hemorrhage (cSAH) is present but with small amounts of blood, the diagnosis of cSAH is not suggested. The arterial vasoconstriction is necessary for the diagnosis. Cerebral angiography was the standard criterion for the detection of cerebral vasoconstriction, but noninvasive techniques such as CT-angiography (Figure 1) (Figure 2) and MRA can be used for the diagnosis and follow-up of RCVS-related vasoconstriction.MRA is increasingly being used and is an effective imaging evaluation for the diagnosis and follow-up (Figures 3‒5).6,7
In most cases CT scan and MRI do not show other intracranial abnormalities.8‒10 In most cases, the RVCS is isolated (Case 1). However, this syndrome may be associated with cSAH (Case 2, Case 3, Case 5), ischemic (Case 4) or hemorrhagic stroke, or PRES. Occasionally, cSAH and infarct, brain hemorrhage and infarct, PRES and cSAH or cerebral hemorrhage may be associated with the clinical-radiological characteristic features of RVCS (Case 5).Some cSAH (30%) are focal SAH, circumscribed to few sulci along the cerebral convexity. 30% of patients present with cSAH with or without brain hemorrhage. CSAH appears on non-contrast CT as a slight sulcalhyperdensity (Figure 4) orhyperattenuation (Figure 1), and on FLAIR as sulcalhyperintensity (Figure 1) (Figure 5). The sensitivity of CT decreases with time, especially if hemorrhage is minimal. FLAIR is more sensitive than CT to depict localized cSAH and the sulcalhyperintensity persists much longer. Susceptibility sequences (T2* or GRE T2 and SWI sequences), may be very useful to confirm the hemorrhagic nature of the sulcalhyperintensity, showing a linear hypointensesignal at the surface of the cortex (ie focal cortical superficial siderosis) (Figure 4). Intracerebral hemorrhagic complications (13-20%) occur early in the course of RCVS (mainly in the first week after onset), whereas ischemic events occur preferentially later (two and three weeks after onset).10,11Hemorrhages are focal and often associated with cSAH. They are rarely localized in the deep structures.12Infarcts are usually localized to watershed areas, and are localized in the carotid or vertebral-basilar territories (Figure 2) and occur a mean of 9 days after onset (Ducros, 2007).The frequency of the RCVS-related infarcts varies among the series, ranging from 6 to 12%.2,7,8
The frequency of the RCVS-associated PRES varies greatly among the series (12%).PRES typically includes acute headache, visual disturbance, seizures, variable focal neurological deficits, altered mental status, associated with a unique and typical clinical-radiological pattern of dominant parieto-occipital vasogenicoedema. Other structures including the brain-stem, cerebellum, basal ganglia, and frontal lobes can be also involved. Minute focal hemorrhages, cSAH and hematoma may occur with the same frequency (15%) in PRES (Figure 5). The incidence of hemorrhagic lesions varies among the series, 20% for Sharma & Kastrup13,14PRES and RCVS share many imaging characteristics, suggesting possible similar mechanisms. Ducros (2007) and colleagues (8) reported that 9-38% of patients with RCVS are associated with PRES lesions. Chen7found that vasoconstrictions are important determinants for PRES and ischemic stroke. More rarely, dissection of the cervical arteries may also be associated with the RVCS.15 These complications (brain infarction, brain hemorrhage, dissection)may cause substantial morbidity and even mortality.8 Distal hyper intense vessels on FLAIR sequence may be present in RVCS (Figure 2), indicating low flow and markers of arterial narrowing. They have been reported as more frequent when ischemic infarcts are present.16 The pathophysiology of RCVS is uncertain. RVCS may be related to a disturbance in the regulation of cerebral arterial tone. This disturbance may be spontaneous or secondary to precipitant conditions or substances, as described previously.
Clinical variants isolated thunderclap headache may bein 75% of cases the single symptom of RCVS and accounts for most benign TCH. However the incidence of isolated headache varies within large published series.7–9 Post-partum RCVS starts within one month from the delivery. In 67% of cases, prior pregnancy has been uneventful. Neurological symptoms begin on day 5 post-partum. In most cases post-partum RCVS has a good prognosis, but fulminant cases and fatal cases have been reported.17–19
Evolution-prognosis
Most patients with RCVS have a good clinical outcome. However, deterioration is not uncommon. Clinical worsening is usually associated with ischemic infarctions and may result in permanent deficits.20
Differential diagnosis
When cSAH is present, the differential diagnosis with aneurysmal SAH (aSAH) is challenging. Patients with aSAH usually present an acute onset and a progressive neurologic worsening. Vasospasm in aSAH is in the vicinity of the SAH, and involves long segments of arteries near the polygone of Willis. Younger age, prior history of headache, lower Fisher SAH grade, more diffuse segmental arterial narrowing, and the presence of bilateral vasoconstriction seem in favor of RCVS rather than of aSAH.21Another differential diagnosis usually evoked is the primary angiitis of the central nervous system (PACNS). In PACNS headaches have not the characteristics of TCH, ischemic infarcts are multiple, associated with subacute cognitive decline whereas MRI reveals old and recent infarcts along with white matter abnormalities. Analysis of CSF is helpful. Post-contrast high resolution MRI vessel wall imaging of the cerebral arteries reveals in PACNS a strong circumferential vessel wall enhancement and thickening, while in RCVS vessel wall imaging shows a diffuse, uniform and minimal wall thickening, corresponding to artery stenosis with mild enhancement, along with total resolution at 3-month control.22–25 Furthermore, MRI can depict other causes of THC, ie, cerebral venous thrombosis and cerebral artery dissection. The primary treatment of patients with RCVS should be the identification and elimination of aggravating factors including drugs (cessation of vasoactive agents), the recommendation of rest, and empirically the administration of drugs targeting vasospasms, calcium channel blockers such as nimodipine and verapamil. This treatment does not affect the course of the disease.3
We present 6 illustrative cases of RCVS that highlight the various presentations of this syndrome.All patients had clinical and neuroradiological follow-up at 3 months, and these controls show the complete disappearance of the clinical and radiological abnormalities In all presented cases, reversal of vasoconstriction was noted on follow-up 3-month MRA.
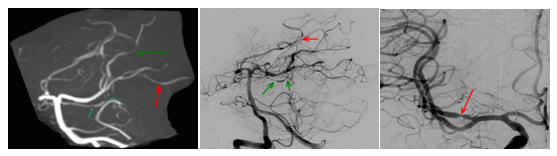
Case 1: A 34-year-old man, cannabis user, presented a TCH. He denied similar headaches in the past. Clinical examination was unremarkable. MRI/MRA showed arterial vasoconstriction, with no SAH nor parenchymal lesion. MRA shows multiple arterial stenosis of proximal and distal branches of the middle cerebral arteries and the vertebral and basilar arteries (Figure 3).
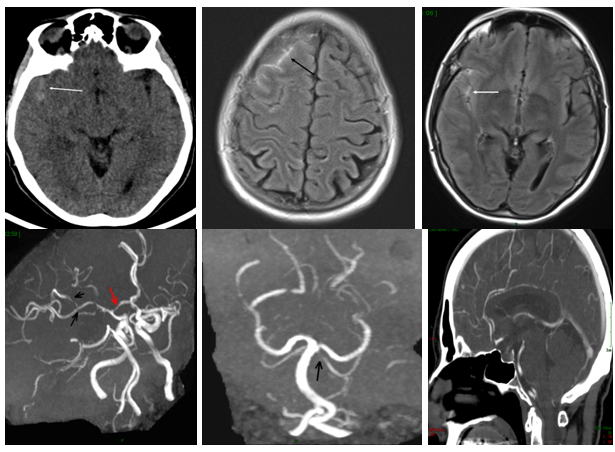
Case 2: A 46-year-old woman with previous history of migraine was admitted to Emergency Department because of a sudden-onset severe headache reaching peak intensity within one minute and associated with nausea. Her medication included Seroplex (10mg daily) and Imigran in case of migraine. Clinical examination was normal. Non-contrast CT showed subtle sulcal SAH which was confirmed on T2* (T2GRE) sequence. MRA showed the multiple segmental narrowing of the cerebral arteries and 3-month MRA follow-up showed the total regression of the vaso-constriction (Figure 4).
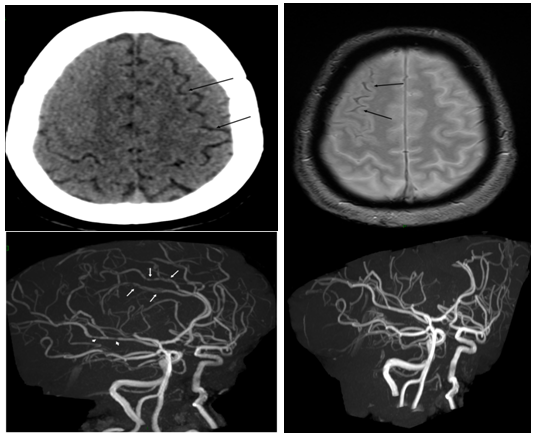
Case 3: A 60-year-old woman with migraine and smoking history presented with thunderclap headache during defecation. The headaches were recurrent during last week, accompanied with vertigo. Her medication included Seroplex. Clinical examination was normal. FLAIR showed sulcal SAH in the right anterior frontal and temporal areas. MRA showed multiple segmental narrowing of the cerebral arteries and 3-month MRA follow-up showed the total regression of the vaso-constriction (Figure 1).
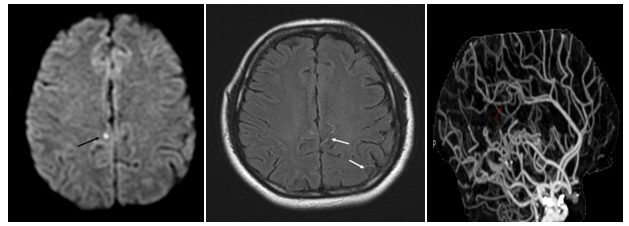
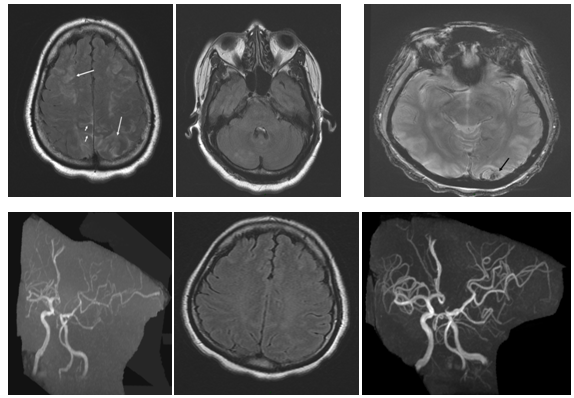
Case 4: A 41-year-old woman presented with acute onset of severe headache and nausea. There was no neurological deficit. There was no previous history of migraine. Previous medication included Seroplex (20mg/day) for depression. Non-contrast CT at admission was unremarkable and ACT showed segmental arterial narrowing. MRI/MRA one day later confirmed the vaso-constriction and revealed dot ischemia on FLAIR and Diffusion images (DWI) with ADC restriction. This ischemic infarct was clinically silent (Figure 2). The outcome was favorable.
Case 5: A 31-year-old woman, gravida 2, presented 2 weeks after caesarean section with headache of progressive onset, phosphenes, rapid decline of visual acuity and vomiting. She had an otherwise uneventful pregnancy. She was found to have an elevated blood pressure of 220/120 mm Hg. Ten hours later the patient developed one episode of generalized tonic–clonic seizure. Emergency MRI showed on FLAIR images extensive vasogenic edema, multifocal sulcal SAH, small right occipital hematoma and extensive vasoconstriction of the cerebral arteries on MRA (Figure 5). These findings were consistent with PRES. The clinical normalisation was observed at day 7, vasogenic edema resolved on 9-day MRI control. Follow-up MRI at 3 months showed a normal aspect of cerebral arteries.
The diagnosis of RCVS should be made on the basis of the typical imaging appearance of cerebral vasoconstriction, in addition to the characteristic clinical onset, and results of CSF analysis. As early diagnosis is crucial to avoid invasive workups and inappropriate treatments, it is critical for emergency physicians to detect the RCVS in patients with TCH, when there is no evidence of aneurysmal SAH, and for radiologists to recognize the imaging pattern of RCVS. Delay in diagnosis may cause clinical deterioration, especially ischemic infarcts and unnecessary diagnostic investigations Furthermore, timely demonstration of complete or near-complete reversibility of vasoconstriction within 3 months, in addition to clinical resolution is useful to confirm the diagnosis.
None.
Authors declare there is no Conflict of interest towards this manuscript.

©2018 Boukobza, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.