International Journal of
eISSN: 2573-2889


Review Article Volume 7 Issue 1
Head of Laboratory Biochemistry and Toxicology, Kiev regional p/n hospital, Germany
Correspondence: Ponizovskiy MR, Head of Laboratory Biochemistry and Toxicology, Kiev regional p/n hospital, Herschelstrasse 33, 90443 Nuernberg, Germany, Tel +49911 6537811
Received: June 01, 2024 | Published: July 9, 2024
Citation: Ponizovskiy MR. The comparison mechanisms of cancer immunotherapy in new method cancer treatment with some modern methods of cancer therapies. Int J Mol Biol Open Access. 2024;7(1):84-101. DOI: 10.15406/ijmboa.2024.07.00175
There were compared the roles of the different methods of Cancer treatments, especially concentrating on advantage of new method of cancer treatment over some negative results appearances of modern methods cancer chemotherapy with large dosage cytotoxic drugs. Thus the current discoveries of influences on the interactions between proteins like CTLA-4, PD-1 and CD-28, CD-80 (B-7-1), CD-86 (B-7-2) on processes for regulation of T cells immune activities that induces to make comparisons to the positive conditions for Cancer immunotherapy versus negative condition for cancer immunotherapy. The immune processes in new method of cancer treatment through Prolonged medical Starvation 42 - 45 days via supporting by extracts of plants with giving very small quantity of cytotoxic substances demand the favourable conditions for concomitant Cancer immunotherapy versus negative conditions of Cancer chemotherapy being created with large dosage cytotoxic drugs. Also there were estimated comparisons of the following methods of Cancer therapies: Surgery therapy, local X-ray therapy, local therapy with radioactive ions. Besides there were shown the influences immune defensive mechanisms on regulatory processes of maintenance stability of Internal Energy in able-bodied organism causing Stationary State of an organism in norm as well as suppression of the influences immune defensive mechanisms on regulatory processes violating maintenance stability Internal Energy of an organism that causes Quasistationary pathologic State of cancer disease metabolic processes. Therefore the favourable condition of mechanism newer method of cancer treatment via “Prolonged medical Starvation (during 42-45 days)” via supporting by herbal extracts induces cancer depression, and using very small dosage weak cytotoxic substances cause destruction cancer cells forming normal condition for operation of immune T cells and macrophages for autophagy promoting best treatment of cancer diseases.
Keywords: warburg effect, T cells, stem cells, cancer cellular cycle, mitosis, meiosis
On the one hand, we study mechanisms of oncogenesis. Viral oncogenes have no own respiratory system, in comparison with the other prokaryotic organisms how different bacteria. Therefore the viral oncogenes use of organism cells’ electron transport chain creating intrusion prokaryotic haploid genome of viral oncogene (v-oncogenes) into nuclear eukaryotic diploid DNA genome of organism’s cells that creates transmutation of affected cells’ nuclear eukaryotic diploid DNA genome by viral oncogene (v-oncogene) haploid accelerating cellular cycle and results in forming accelerating cellular cycle of cancer cells’ combined haploid-diploid DNA genome. Just using organism cells’ electron transport chain for viral cellular oxidative processes, cancer cells use electron transport chain for cancerous combined nuclear Meiosis-Mitosis DNA genome exerting oxidative processes of electron transport chain increased production ATP which induces anaerobic oxidative phosphorilation in Glycolysis (Figure 1).1 Then Glycolysis energy is divided in nodal point of Acetyl-CoA bifurcations on anabolic endergonic biosynthetic processes and catabolic anaerobic exergonic oxidative processes [NPBac] in norm.1 Versus normal metabolic processes, there occurs the excessive increased anabolic endergonic biosynthetic processes with consumption great quantity energy for accelerated cancer cellular cycle in cancer tissue. The excessive increases anabolic processes with the enormous consumption of energy and Acetyl–CoA for anabolic biosynthetic processes in cancer tissue leads to insufficient energy and Acetyl-CoA for metabolic processes of an organism that results in the overload of “nodal point of bifurcation anabolic and catabolic processes” [NPBac] and suppression catabolic anaerobic exergonic processes because of the lack of Acetyl–CoA for catabolic anaerobic oxidative phosphorilation processes of Krebs tricarboxylic acids cycle in cancer tissue (Figure 1).1 Excessive increase of lactic acids production is the necessary marker of Glycolysis for endergonic mechanism accumulation of energy for huge anabolic biosynthetic processes in condition Glycolysis metabolism and enormous consumption of energy for anabolic biosynthetic processes in cancer tissue which is driving mechanism of metastasis.1,2 Hence the suppression catabolic anaerobic exergonic oxidative phosphorilation processes of Krebs cycle leads to expression catabolic aerobic exergonic oxidative processes showing shift balance catabolic anaerobic oxidative processes & catabolic aerobic oxidtive processes into increased catabolic aerobic oxidative processes in cancer tissue. Thus excessive anabolic endergonic biosynthetic processes via increased Lactic acids as Glycolysis marker and expression catabolic aerobic oxidative processes creates oxidative Glycolysis of Warburg effect as phenomenon of Cancer tissue metabolism versus Pasteur effect of incompatibility oxidative processes with Glycolysis as phenomenon of healthy tissue metabolism.1 On the other hand, we study the condition for mechanism of cancer immunotherapy through comparison the roles of modern methods of Cancer Therapies and of new method of cancer treatment. The article studied different conditions for driving mechanism Cancer immunotherapy considering influence of the interactions between proteins CTLA-4, PD-1 and CD-28, CD-80 (B-7-1), CD-86 (B-7-2) on processes regulation of immune T cells activity for maintenance stability Internal Energy of an organism in norm and in cancer pathology. Also it was considered the influences of the interactions between proteins CTLA-4, PD-1 and CD-28, CD-80 (B-7-1), CD-86 (B-7-2) on processes regulation T cells functions showing suppression cancer development.3–14 Viral oncogene affected nuclear DNA of cells induce suppression of reticulo-endothelial system (RES), thereby it causes the suppression of T cells activity due to suppression in producing immunoglobulins like CTLA-4 and PD-1, which result in expression production of supplemental quantity of immunoglobulins like CD-28, CD-80 (B-7-1), CD-86 (B-7-2) in cancer tissue (15, 16). The article elucidates as the absence of production supplemental quantity of both immunoglobulins like CTLA-4 and PD-1 and CD-28, CD-80 (B-7-1), CD-86 (B-7-2) in condition of modern methods cancer chemotherapy with large dosage cytotoxic drugs. Therefore the condition of modern methods cancer chemotherapy prevents the operation of cancer immunotherapy versus the condition of the mechanism arisen production of immunoglobulins like CTLA-4, PD-1 through overcome production supplemental quantity of immunoglobulins like CD-28, CD-80 (B-7-1), CD-86 (B-7-2) in new method cancer treatment that gives possibility to use cancer immunotherapy with autophagy for successful termination of cancer therapy.
Considering mechanism maintenance stability Internal Energy of an open non equilibrium non-linear thermodynamic system of an organism, it must show regulative mechanism of an organism which promotes stability Internal Energy of Stationary State thermodynamic system of an organism in norm. There is the equation of the first law of thermodynamics:
H = U + Wint + Wext [H, Enthalpy (Common Energy); U, Internal Energy; Wint , Internal Work; Wext, External Work
Just Internal Work (Wint) causing mechanism maintenance stability Internal Energy (U) of an organism both in norm and pathology occurs via biochemical mechanism of three levels regulation of mechanism stability Internal Energy [highest level regulation [Central Nervous System], high level regulation [“Equilibrium Constant of ionic metabolism”, “Equilibrium Constant of acid –- alkaline metabolism”, “Equilibrium Constant of oxidative – reduction Potentials of metabolism” and “Equilibrium Constant of coagulating system of blood”], low level regulation [“Equilibrium Constant of energy exchanges” and “Equilibrium Constant of metabolism”] (Figure 2).17–19 Stationary State of open non-equilibrium non-linear thermodynamic system of an able-bodied organism is characterized by stability of Internal Energy [the temperature 36,0°C – 36,9°C by which all enzymes operate, stable index pH=7,35 in blood and in neurolymph etc.] and Internal Medium [stable concentrations of substances in blood and neurolymph] according the first law of thermodynamics. Stationary State of open thermodynamic system of an able-bodied organism displays balance catabolic exergonic processes & anabolic endergonic processes corresponding to low level maintenance stability Internal Energy of an organism. Quasi-stationary States of an open thermodynamic system of a sick organism on inflammatory processes is characterized by shift of balance catabolic processes & anabolic processes into excessive catabolic exergonic processes. Quasi-stationary States of a sick open non-equilibrium non-linear thermodynamic system of an organism on cancer disease is characterized by shift of balance catabolic processes & anabolic processes into excessive anabolic endergonic processes displaying Warburg effect mechanism.1,18,19 Also interdependence related cellular cytoplasms of chemical potentials (µ) in an organism’s cells exerts related cellular resonance waves of an organism’s cells due to cellular capacitors of cells’ receptors operations which induce biophysical mechanism maintenance stability Internal Energy (U) of an organism both in norm and pathology (Figure 3).20–23 Besides biochemical mechanisms maintenance stability Internal Energy (U) of an organism in norm and pathology and biophysical mechanisms maintenance stability Internal Energy (U) of an organism in norm and pathology are subjected to regulative mechanisms of hormonal systems as well as defensive role of both humoral immune system and cellular immune mechanism via phagocytosis against environmental influences which are supplemented with autoimmune reactions of autophagy for cleaning functions of Internal Energy (U) in an organism by reticuloendothelial system with macrophages and monocytes.24 These mechanisms of maintenance stability Internal Energy (U) of an organism are operated in norm and in cancer pathology.
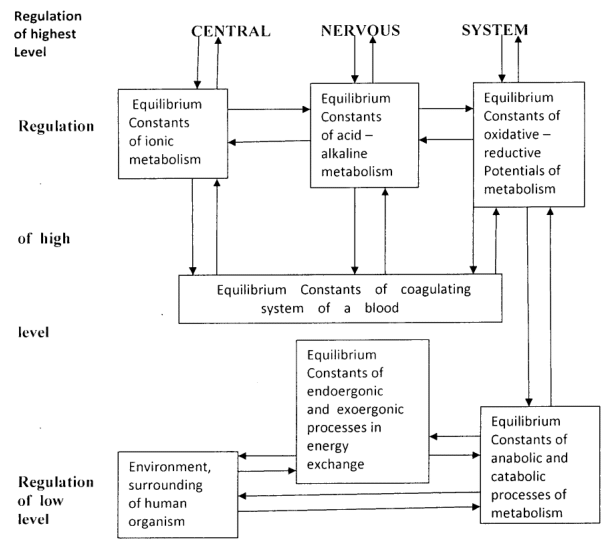
Figure 2 The mechanism of maintenance stability of internal energy and internal medium an organism.
Footnotes: Metabolic and energy “Equilibrium constants” regulate interactions of intracellular and extracellular chemical potentials (µint ⟷ µext) for maintenance stability of Internal energy and Internal medium an organism. The intracellular and extracellular chemical potentials (µint and µext) cause the formations of the positive/negative charges on internal and external membranes of cellular wall, prompting operation of remote cellular reactions cellular capacitors operation.
The therapeutic activities of modern methods of cancer treatment are insufficiently occurred against the etiologic targets of cancer agents that sometimes gives chance for surviving of some oncological viruses creating relapse cancer disease in opposed to the offered new method cancer treatment. Modern methods of cancer therapy target the cancerous cells as the ill elements which are affected by viral oncogene. There are the some modern methods of cancer therapy unlike the offered new method cancer treatment:
1) Surgery cancer therapy via operation of the oncologic tumor is the therapeutic activity of removing cancerous focus which requires early operation of the cancer tumor due to absent unoperative metastasis, cahexia and other cancerous neglected states. However it is occurred late visit to doctor of cancer sick person that makes impossible efficient surgical removing cancerous tumors because of appeared inoperable metastases. Therefore the Surgical palliative operation eliminates cancerous symptoms which are dangerous for life of sick man which makes some prolonging his life.
2) The Chemotherapy with large doses of cytotoxic drugs ruins cancer cells and also suppress normal immune and hormonal cells of an organism leading to suppresion immune and hormonal systems of an organism. The purposes of the modern methods of Chemotherapy with large doses of cytotoxic drugs are either ruining cancer tumor with its cancer cells or prevention cancer tumor growth and metastasis that prevents palliative chemotherapy of only reducing cancerous symptoms.
3) Roentgenotherapy and Radiotherapy targets cancerous tumors, ruining cancer cells affected by viral oncogene and suppress immune and hormonal systems of an organism.
4) The offered new method cancer treatment targets Warburg effect causing suppression of cancer metabolic proceeses due to suppression of oncological viruses activities and expression anticancer activities of immune and hormonal systems which leads to destruction cancer cells together with oncoviruses by affected with the very small quantity of cytotoxic substances. Further the dead cancer cells were ruined by an organism’s immune system, and the infected microRNA from the ruined dead cancer cells were removed by autophagy for cleaning Internal Medium of an organism (see below). Thus activity of immunotherapy against pathologic v-oncogenes makes efficient cancer treatment. Thus the offered new method cancer treatment causes efficient cure of cancer disease. This cure of cancer disease is occurred by offered new method cancer treatment because of without weaknesses of both Chemotherapy with large doses of cytotoxic drugs and Roentgenotherapy or Radiotherapy creating suppressed anticancer immunotherapy (both humoral immune system and cellular immune systems).25,26
The using therapeutic targets in modern methods cancer treatment
The targets of modern methods cancer disease treatment are either oncogenesis mechanisms or separate links of the cancer metabolism. Consequence of modern methods chemotherapy in cancer therapy is following: Protective functions of an organism are violated because the large dosages cytotoxic drugs damage as cancer disease focus as well as mechanisms of immune system and hormonal systems of an organism. Using modern Methods Cancer Treatzment as target of affected DNA by v-oncogene, it is leads to suppression cancerous proliferative processes of cancer cellular cycle in which combined pathologic haploid-diploid cancer cellular cycle with prevalence haploid link transits into human normal prevalence diploid accelerating cellular cycle of diploid-haploid cancer cellular cycle. However defensive mechanisms of immune system and hormonal systems are subjected to suppression their activity by large dose cytotoxic drugs in an organism too. Thus these methods of cancer therapy make negative influences especially on immune system and hormonal systems that decreases efficiency of cancer therapy considerably because of decreasing defensive mechanisms of an organism. It creates sometimes the resistanse to cytotoxic drugs after obtaining intensive therapy with large dose cytotoxic drugs leading to survived of some depressed oncoviruses activity that can even lead sometimes to relapse cancer disease.25–28 Also the cancer cells fall into apoptosis of their destruction into cancer cells‘ death due to modern methods of cancer treatment. However dead cancer cells contain the substance of the viral oncogenes (v-oncogenes) molecular structures infected these dead cancer cells. These molecular structures of the viral oncogenes (v-oncogenes) are fallen away as microRNAs into external medium when these dead cancer cells are ruined by immune T cells activities resulting sometimes in the relapse cancer disease (see below). Lopez-Lazaro has correctly noted “According to the most accepted models of carcinogenesis, the alterations of DNA tumor cells are targets for cancer therapy. However the high number variability of these DNA alterations are obstacles for the design of gene-based therapies that may have a major impact on cancer mortality”.29 There are occurred the replacement atoms in nuclear DNAs in cancer cells from oncologic Meiosis-Mitosis into oncologic Mitosis-Meiosis with expression Mitosis over suppression Meiosis phase of cancer cellular cycle by such cytotoxic drugs as fluorouracil, gemcitabine (gemzar) and the others. Thus such replacements atoms in nuclear DNAs treating by large doses of cytotoxic drugs fluorouracil, gemcitabine or other analogues of pyrimidin cause suppression DNA replication of cancer cells maybe in greater degree than of an organism’s cells, especially in immune and hormonal systems.29–32 Besides another target of fluorouracil, gemcitabine or other analogues of pyrimidines is the enzyme ribonucleotide reductase [RNR].30–32 The diphosphate analogue binds to RNR active site and inactivates the enzyme irreversibly. Once RNR is inactivated, the cell cannot produce the required deoxyribonucleotides for DNA repair, and cancer cells are fallen into apoptosis.30–32 Inhibition of RNR leads to destruction of the function mismatch repair proteins (MMR) for nDNA reparation which are generated by nine genes of MMR function and among them the main five genes of mismatch repair proteins (MMR) function (MLH1, PMS1, PMS2, MSH2, and MSH6),33–35 i.e. destruction of driving mechanisms of anabolic processes in S/G2/Meiosis-Mitosis phases of cellular cycle, which cause destruction replicative processes of tumor growth. However these methods also some violate the driving mechanisms of anabolic processes in the organism’s cells, some influencing on cells especially of immune system and hormonal system negatively causing some suppressing defensive functions of the organism. Lopez-Lazaro has also suggested that cancer cells can be killed by increasing the cellular level of H2O2 and/or by attenuating glycolysis due to using prooxidant agents (paclitaxel, cisplatin, doxorubicin, arsenic trioxide, bortezomib, procarbazine and etoposide) and glycolysis inhibitors (2-deoxy-D-glucose, lonidamine, 3-bromopyruvate and dichloroacetate).29 The role of glutathione and thioredoxin antioxidant systems in detoxifying ROS and preventing redundancy of H2O2 especially in cancer tissue are not sufficient modes of cancer therapy. Also Lopez-Lazaro has noted inefficiency glycolysis inhibition therapy without use of drugs against increasing the cellular level of H2O2.29 Besides the Lopez-Lazaro note can be explained by the mechanism of excessive H2O2/Free radicals activity which exerts accelerating cellular cycle of cancer cells’ replications via realizing 2nDNA reaction causing neutralization H2O2.29 Besides it is occuured inhibition of glycolysis function generating energy for oxidative phosphorilation and distributing energy between anabolic and catabolic processes (Figure 1).1,30–36 Just glycolysis inhibition by cancer therapy with large quantity cytotoxic drugs suppress interacting between catabolic anaerobic processes and the function of mitochondrial oxidative aerobic mechanism which impact on as excessive anabolic processes as well as catabolic anaerobic processes Krebs cycle (TCA) in cancer tissue. Therefore viral oncogenes of haploid cellular cycle switch over glycolysis oxidative phosphorilation into mitochondrial aerobic oxidative function in order to consume energy from the energy generation by the proton pumps of the electron transport chain to Complex V ATP production by ATP synthase of the mitochondrial oxidative membranous mechanism, according famous Mitchell P.D. theory chemiosmotic rationale of vectorial Graphics, for oncologic viruses survival.36 Just use the mitochondrial oxidative membranous mechanism of redundancy H2O2 promotes suppression of viral oncogene by cancer therapy with large quantity cytotoxic drugs. However inhibition glycolysis leads to inhibition Krebs cycle of tricarboxylic acid (TCA) for oxidative phosphorilation and inhibition anabolic endergonic biosynthetic processes in organism’s cells, especially cells of immune and hormonal systems.37 The inhibition of the main energy generators both Glycolysis and Krebs cycle of tricarboxylic acids TCA with destruction of mitochondrial oxidative membranous mechanism and other cellular membranous mechanisms by redundant H2O2 influences also negatively on defensive functions of an organism due to violation of cells’ hormonal and immune systems, to a larger or lesser degree by the large dosage of these cytotoxic drugs.
According to data Bonnet et al,37 dichloroacetate (DCA), an inhibitor of Pyruvatdehydrokinase Complex (PDK), changes cancer cell metabolism via shifting pyruvate metabolism from the cytoplasm-based glycolysis to the mitochondria-based glucose oxidation that promotes decrease of lactate production, increase of reactive oxygen species (ROS) production, and decrease of negative mitochondrial membrane potential (∆Ψ m).38 Hence it occurs the increase of mitochondrial catabolic aerobic oxidative processes promoting decreased negative mitochondrial membrane potential (∆Ψ m) which is considered as the positive effect. Also, DCA promotes the inhibition with downregulation of Kv channels that result in efflux of K+ and decreased intracellular K+, leading to apoptosis of cancer cells, i.e., pro-apoptotic effect of DCA. DCA effectively decreases tumor growth and then leads to cancer cells’ death. The shift of pyruvate metabolism from the cytoplasm-based glycolysis to the mitochondria-based glucose oxidation, induced by DCA, promotes the activation of mitochondrial catabolic aerobic oxidative processes with consumption energy, that suppress anabolic endergonic processes because of lack of energy and Acetyl-CoA for anabolic processes, and decrease tumor growth (Figure 1).1,25,26 Indeed the increase of mitochondrial catabolic aerobic oxidative processes stimulates production of ROS, and great excess of ROS can ruin mitochondria, i.e., pro-apoptotic effect of DCA. However the same effect of DCA activity on cancer metabolism can also affect normal healthy cells of the hormonal system and immune system although to some lesser degree.
An increase of oxidative metabolism, induced by the mitochondrial protein named frataxin, inhibits cancer growth, according to the research of Schulz et al.39 Taking into account that cancer growth results in arising huge anabolic processes into G1/S/G2 phases of the cellular cycle, the mechanism of operation of frataxin provides the shift from the excessive anabolic pathway into expression catabolic aerobic oxidative pathway.1,25,26 However, such action of frataxin is directed as towards cancer cells as well as towards normal healthy cells of hormonal and immune systems.
Antitumoral activity of the cytotoxic agents known as azatoxin, an inhibitor of tocoisomerase II and tocoisomerase I, is based on destruction of the development anabolic pathway.1,25,26,40–42 The polymerization processes are one components of the anabolic pathway. However, such destruction of the cancer anabolic pathway also affects normal healthy cells of the hormonal and immune systems.
Nghiem et al. have described the four major targets of protein kinase classes:43
1) promitotic kinases (epidermal growth factor of receptor tyrosine kinases including Her2/neu/ErbB2 and cyclin-dependent kinases); 2) proangiogenic kinases, which are required for the increased vascular supply energy required by tumors growth via vascular endothelial growth factor (VEGF) and platelet-derived growth factor receptor kinases; 3) DNA replication checkpoint kinases (ATR and Chk-1) that normally prevent entry into mitosis if replication is inhibited by DNA damage or insufficient nucleotides. These kinases appear to be more important for cancer cells than for normal cells; 4) kinases involved in nutrient sensing and regulation of metabolic pathways (FRAP/mTOR and Her2).43
The first class of promitotic kinases targets are elements of the cellular membranous apparatus regulating processes of substance transport through cellular membranes and states of equilibrium of alternating balance chemical potential of intracellular medium (µint) & (µext) chemical potential of extracellular medium, i.e., driving mechanisms of moving processes in G1/ S/ G2 and Meiosis-Mitosis phases of cancer cellular cycle, corresponding Prigogine Theorem and Glansdorff & Prigogine theory.26,43 These targets are attacked by the cytotoxic drugs which influence on G1/ S/ G2 and Meiosis-Mitosis phases of cancer cellular cycle and processes cell reproduction. The variety of epidermal growth factors are the receptors tyrosine kinases including Her2/neu/ErbB2 of cellular membranes which are selectively bound to the ligand forming receptor-ligand complex which influence on equilibrium chemical potentials between intracellular medium and an extracellular medium via regulating processes of substance transport through cellular membranes.44,45 Then the separated ligands provide the cell with necessary substances that enhances anabolic processes in the G1 phase of the cellular cycle. There are the drugs which act against these targets in cancer therapy: the antibody-directed medicine against Her2 are named Herceptin (trastuzumab), 2C4 cetuximab (IMC-C225).43 In addition Nghiem et al. describe the negative side effects of these drugs on heart activity. The other drugs, Gleevec (STI-571 or imatinib mesylate) is the promitotic kinase class which target is the BCR-ABL oncogene. Gleevec (STI-571 or imatinib mesylate) is a tyrosine kinase with aberrant regulation of the kinase domain derived from the normal cellular Abl tyrosine kinase. The BCR-ABL oncogene is produced in chronic myelogenous leukemia (CML). Gleevec is the selective inhibitor of BCR-ABL oncogene. However some authors note that the resistance to frequent use of the drug arise the mild allergic effects such as skin rash and edema. The subsequent development of cellular cycle takes place via cyclin-dependent kinases (CDK2, CDK4, CDK6), which can be inhibited by Flavopiridol (alvocidib). Also Flavopiridol is the inhibitor of protein kinase A and EGF receptor tyrosine kinase. Flavopiridol shows activity against gastric and renal cancers in early clinical stages. Nghiem et al. note that these drugs are cytostatic rather than cytotoxic.43 Therefore these drugs inhibit promitotic kinases causing inhibition the processes in G1/ S/ G2 and Meiosis-Mitosis phases of cancer cellular cycle in cancer cells as well as in cellular cycle of normal cells, including cells of immune and hormonal system, to a lesser degree than cancer cells. As concerning cytostatic properties rather than cytotoxic property of these drugs, it is meant that these drugs are weaker than v-oncogenes for giving possible viral oncogene survival. Besides these therapeutic drugs can reduce resistance to viral oncogene of the normal cells, expecially hormonal processes and immune processes.
The second class of proangiogenic kinases targets are the VEGF family of transmembrane tyrosine kinase receptors, which are presented by three subtypes of receptors bound in different combinations to seven ligands. One VEGF receptor is KDR (VEGF-R-2/FLK1), which is important for endothelial cell proliferation and angiogenesis, both of which promote tumor progression.43 It was shown that a small molecule called SU-5416 specifically inhibits this target.43 Subsequently, it was found that another small molecule called SU-6668 targets the catalytic activity of a wide variety of tyrosine kinases including all three VEGF receptors: PDGF, FGF, and c-kit.43 The antibody-fusion protein bevacizumab (Avastin) is a monoclonal antibody that binds the VEGF ligand and prevents it from interacting with the VEGF receptor on vascular endothelial cells.43 Also, it was shown that both Rapamycin, and the closely related Rapamycin ester CCI-779, influence on the intracellular chemical potential (µ) leading to suppression the proliferation in endothelial cells due to changed resonance waves of VEGF receptor‘s variable capacitors.43,59 However, these drugs cause inhibition as the cancerous ligand and VEGF receptor, as well as normal ligand and normal VEGF receptor, preventing normal interactions of VEGF receptor/VEGF ligand as well as pathologic interactions of VEGF receptor/VEGF ligand, reducing as hormonal cells as well as reactance and immune resistance of an organism.
The third class: The mutation of DNA replication checkpoint kinases (ATR and Chk-1) is inhibited by a small molecule known as UCN-01. Also the normal DNA replication checkpoint kinases (ATR and Chk-1) prevent entry into mitosis. Thus the normal replication is inhibited because of blocked DNA or insufficient nucleotides also causing some inhibition hormonal processes and immune processes.43
The fourth class: The kinase involved in nutrient sensing is a large protein kinase known as FRAP (mTOR/RAFT1), which is inhibited by complex FKBP12-Rapamycin [Previously, Rapamicin (immune suppressant and simulteneously anticancer agent) bind a small cellular protein called FKBP12].43 Then the rapamycin ester CCI-779 (cell cycle inhibitor) appears, which alters stability cellular cycle. Then it is occurred that the normal cellular role of FRAP/mTOR is served as a nutrient sensor, regulating transcription and translation for optimal response to abundance or paucity of nutrients such as amino acids and glucose.43 Then it was discovered that the loss of a tumor suppressor called Pten (phosphatase and tensin homolog) is characteristic of cancer cells with rapamycin sensitivity. Therefore Pten has now been established as an important tumor suppressor. Hence these Pten-deficient in tumor cells are far more sensitive than normal cells to this agent.43 So, it may be assumed that protein kinase FRAP/mTOR as well as Akt and p70S6 kinase operate in the anabolic pathway, but Pten (phosphatase and tensin homolog) operates in the catabolic anaerobic oxidative pathway of metabolism, suppressing the anabolic pathway (Figure 1). Therefore, the inhibitory capacity of rapamycin ester CCI-779 can suppress anabolic processes of FRAP involved in nutrient sensing in mutant cancerous cells as well as in normal cells, reducing immune resistance as well as hormonal processes of an organism. However, Pten defends catabolic anaerobic processes from actions of rapamycin CCI-779 and is also an important tumor suppressor. However all these targets are also driving mechanisms immune and hormonal cells which are affected by action cytotoxic drugs.
Gogvadze et al. studied mechanisms destruction of apoptotic resistance in anticancer therapy and noted that mitochondria are a preferable target for such therapy, operated via suppression of mitochondrial aerobic oxidative function.46 All-trans Retinoic acids (ATRA), being a natural derivative of vitamin A, disrupt mitochondrial aerobic function and cause cell death, according to both Gogvadze45 and Schmidt-Mende.47 Gogvadze et al.46 and Neuzil et al.48 described α-tocopheryl succinate (α-TOS) (analog of vitamin E), a selective cancer cell killing agent, and pro-apoptosis effect of α-TOS via interaction with Complex II of the mitochondrial aerobic respiratory electron transport chain.46,49,50 In addition, Gogvadze et al. and Neuzil et al. described the molecular mechanism of induced apoptosis in cancer cells and stimulation of ROS production.46,50 Gogvadze et al. and Yu et al. described the α-TOS pro-apoptotic effect via Bax translocation from the cytoplasm to the mitochondria.46,51 Also, Gogvadze et al. and Wolvetang et al. noted that anti-apoptotic protein Bcl-2 does not block apoptosis, which is induced by respiratory electron transport chain inhibitors of mitochondrial DNA.46,52 Thus, these researchers have detected the dependence on interactions of pro-apoptotic and anti-apoptotic agents in the mitochondrial aerobic respiratory electron transport chain operation, which promotes aerobic oxidative function of mitochondria. Suppression or blockage of the mitochondrial aerobic respiratory electron transport chain promotes cellular death (apoptosis) since the mitochondrial aerobic respiratory electron transport chain participates in forming of the catabolic exergonic oxidative pathway of cellular metabolism, creating the greatest quantity of calories for maintenance stable temperature (36.0°С – 37.5°С) at which all enzymes operate in an organism.60 Therefore Engel and Evens research of oxidative stress employs in anticancer therapy via stimulation of redundant ROS production and/or depletion of protective reducing metabolites [e.g. glutathione (GSH) and NADPH], shows that destabilization of mitochondria can cause cell death (Apoptosis).46,53 Examinations of the chemotherapeutic agent arsenic trioxide, which stimulates redundant ROS production [by Engel and Evens interpretation] and leads to apoptosis through Bax- or Bak-dependent mechanisms, confirm the mechanism of apoptosis as a result of disturbance of mitochondrial functions.46,54,55 However, the normal healthy cell can also be subjected to disturbance of mitochondrial functions, causing violation of the mitochondrial catabolic aerobic respiratory electron transport chain of cells in hormonal and immune systems, reducing reactance and immune resistance of an organism. Indeed normal function any cells [both normal and pathologic cells] depend on interactions between cellular chemical potential (µinner cell) and extracelluar chemical potential (µouter cell) which is formed by interacting combined balances catabolic aerobic processes & catabolic anaerobic processes & anabolic processes of intracellular and extracellular mediums.56,57 These interactions between intracellular medium and extracellular medium are the mechanisms of stability Internal Energy of each cell as stable basophilic chemical potential cytoplasm via staining cells.60 Just some cytotoxic drugs replace one of building block of nucleic DNA in which hydrogen atoms are replaced by fluorine atoms. Also estrogen cellular receptors (ER) are used as the targets for some anticancer drugs. Just Tamoxifen is used as prodrug, having relatively little affinity to the estrogen receptor (ER).58 It is metabolized the cytochrome P450 in the liver by isoform CYP2D6 and CYP3A4 into active metabolites such as 4-hydroxytamoxifen (4-OHT) (afimoxifen) and N-desmethyl-4-hydroxytamoxifen endoxifen which have 30–100 times more affinity with the ER than tamoxifen itself.31 These active metabolites compete with estrogen for binding to the ER. In breast tissue, 4-OHT binds to ER as an antagonist of estrogen so that transcription of estrogen-responsive genes is inhibited.58 Tamoxifen has 7% and 6% of the affinity with estradiol for binding to the ERα and ERβ, respectively, whereas 4-OHT has 178% and 338% of the affinity with estradiol for binding to the ERα and ERβ.31 4-OHT binds to ER, forming the ER/tamoxifen complex which recruits other proteins known as co-repressors and then binds to DNA in order to modulate gene expression.58 Some of these proteins include NCoR and SMRT. Tamoxifen function can be regulated by a number of different variables including growth factors.58 Tamoxifen needs to block growth factor proteins such as ErbB2/HER2 because high levels of ErbB2 shown tamoxifen resistant cancers. Tamoxifen seems to require a protein PAX2 for its full anticancer effect.58 In the presence of high PAX2 expression, the tamoxifen/ER complex is able to suppress the expression of the pro-proliferative ERBB2 protein. In contrast, when AIB-1 expression is higher than PAX2, tamoxifen/ER complex upregulates the expression of ERBB2 resulting in stimulation of breast cancer growth.58 4-OHT binds to ER competitively (with respect to the endogenous agonist estrogen) in tumor cells and other tissue targets, producing a nuclear complex that decreases DNA synthesis and inhibits estrogen effects. It is a nonsteroidal agent with potent antiestrogenic properties which compete with estrogen for binding sites in breast and other estrogen tissues. Tamoxifen causes cells to remain in the G0 and G1 phases of the cellular cycle because it prevents cancerous cells from dividing but does not cause cell death. Thus tamoxifen is cytostatic rather than cytocidal. N,N-Didesmethyl-4-hydroxytamoxifen (norendoxifen), another active metabolite of tamoxifen, has been found to act as a potent competitive aromatase inhibitor (IC50= 90 nM), and may also be involved in its antiestrogenic activity.58 Really such replacement estrogen receptors with molecular relative affinities drugs as non operating element disrupts cellular mechanisms of passive and active transport across cellular membranes due to violation interaction between intracellular chemical potential (µinner cell) and extracellular chemical potential (µouter cell) causing critical violation of cellular capacitors operations leading to suppression activity as resonance waves of cancer cells’ capacitors as well as resonance waves of an organism’s cells’ capacitors especially of sexual hormonal glands cells’ capacitors. Such suppression mechanisms sexual hormonal processes in cancer tissue and in an organism inhibits anabolic endergonic processes as in cancer cells as well as in able-bodied cells of an organism reflecting suppression sexual hormonal processes and immune defensive processes.59–62
Mechanisms of resistance cancer cells to cytotoxic anticancer drugs and relapsed cancer disease after intensive cytotoxic therapy of modern methods cancer therapies
Modern metods cancer therapy use following targets: cancer tumors, cancer cells, cancer cells’ nucleus and its DNA, cancer cells’ mitochondria, cancer cells’ organelle as well as links between them. Fedier A. et al. studied DNA minor groove binded by Brostallicin (PNU-166196) causing cytotoxic effect on cancer tumor activity with its cancer cells and also retaining sensitivity to Brostallisin in deficient of DNA mismatch repair proteins functionas, i.e. sensitivity to Brostallisin does not depend on DNA MMR function.61 However the intensive cellular cycles of immune cells and hormonal cells need DNA repairing especially. Just phagocytosis requires permanent new immune cells forming by reticuloendothelial system (RES) and by marrow, as well as producing hormones by hormonal glands are required reparations both for an organism cells’ cellular cycles and for cancer cells’ accelerating cellular cycle. Therefore DNA minor grooves of immune cells and DNA minor grooves of hormonal cells are also binded by Brostallicin (PNU-166196) although normal cells in less rate than cancer cells. Further violation immune and hormonal function of an organism causes common disbalance anabolic endergonic processes & catabolic anaerobic exergonic processes & catabolic aerobic exergonic processes of Quasi-stationary pathologic State of an organism.
Besides oncogenesis can leads as to resistance cancer cells to cytotoxic anticancer drugs as well as to relapsed cancer disease after intensive cytotoxic therapy due to following mechanisms. Just resistance cancer cells to cytotoxic anticancer drugs and relapsed cancer disease are occurred after some times of intensive chemotherapeutic treatment with large dosage cytotoxic drugs which affect nuclear DNA (nDNA) genome suppressing Meiosis-Mitosis phase cancer cellular cycle leading to apoptosis of some cancer cells death. Suppression prokaryotic genome of Meiosis-Mitosis phase cancer cellular cycle also touch on suppression both eukaryotic cells‘ DNAs genomes of Mitosis phase in immune and hormonal systems [in less ratio than cancer cells] resulting in suppression immune and hormonal systems. Therefore it is occurred resistance between antiviral force of drugs and oncologic viral force for survival some viral oncogenes and retaining infected by v-oncogenes of microRNAs from destructed dead cancer cells in conditions of suppression of reticuloendothelial system, macrophages and monocytes which create autophagy of dead cells for cleaning Internal Energy of an organism as well as suppression both immune and hormonal systems. Thus the survived viral oncogenes with retained infected by v-oncogenes of microRNAs from destructed dead cancer can arise relapse cancer disease if there are suppressed reticuloendothelial system, macrophages and monocytes.
Also the survived viral oncogenes retaining Meiosis-Mitosis phase reflects prevalence oncologic viral force for viral oncogenes survival in which viral oncogenes [v-oncogenes] use rest suppressed hormonal cells (not damaged by cytotoxic drugs) for supporting cancer cells development via accelerating cellular cycle.62,63 Besides rest some suppressed hormones activity of hormonal glands and immune T memory cells, T helper cells, T killer cells with B cells are restored after some time of intensive cytotoxic drugs treatment in which hormones activity restore immune T memory cells, T helper cells, T killer cells. The immune T memory cells learn molecules of cytotoxic drugs into hormonal gland’s cells injured by these cytotoxic drugs. Then T memory cells transmit these data to T helper cells and further to T killer cells. The T killer cells destruct these cytotoxic drugs causing resistance to these anticancer drugs. As concerning used molecular structure cytotoxic drugs in some hormone activity, this must be explained by the following example: In breast cancer cells after intensive cytotoxic therapy it is occurred either resistance to cytotoxic drugs or relapse cancer disease because cytotoxic drugs suppress cancer Meiosis-Mitosis cellular cycle and simultaneously suppress as activity immune T memory cells, T helper cells, T killer cells as well as activity female Estrogens [estrone, estradiol, estriol] and Progesterone hormones. After some time from intesive chemotherapy, it is begun expression as restored immune T memory cells, T helper cells, T killer cells as well as activity female estrogens [estrone, estradiol, estriol] and Progesterone hormones in which restored activity of estrogens can prevail over restored activity of progesterone causing shift balance estrogens & progesterons into expression estrogens. Therefore estrogens [estrone, estradiol, estriol] either restore activity of suppressed cancer cells after some time of chemotherapy inducing relapse cancer disease or exert expression as restored immune T cells in which T memory cells learn molecule of cytotoxic drugs into hormonal gland’s cells injured by these drugs. Then T memory cells transmit the data of molecules cytotoxic drugs to T helper cells and further to T killer cells. The T killer cells destruct these cytotoxic drugs causing resistance to these anticancer drugs. Thus excessive estrogens [estrone, estradiol, estriol] operate how carcinogens. The balance sexual hormons estrogens & progesterons induce normal development female organism from pubertal age till climacteric age where progesteron is expressed. On the other hand, often molecules of some cytotoxic drugs don’t leave data of theirs molecular genomes in substances of hormonal glands‘ injured cells, and T memory cells don’t learn their molecules. Therefore these cytotoxic drugs are not subjected to destruction of their molecules by T killer cells. Thus there are not arisen of resistance to anticancer activity of these cytotoxic drugs.
Footnotes concerning different cytotoxic drugs:
Meyers M. et al. studied mechanism cytotoxic action Fluoropyramidine causing damage processes biosynthesis proteins via transcription and translations DNA processes requiring mechanism of DNA reparation via operation DNA mismatch repair proteins function. Author did not find differences sensitivity to Fluoropyramidine between MMR-deficient cancer cells and MMR-proficient cancer cells.64 However Fluoropyramidine causes also violation processes biosynthesis immune antibodies by immune system and hormones by hormonal glands. Thus damage biosynthesis of proteins causing by Fluoropyramidine violates immune and hormonal cells’ functions although in less rate than cancer cells. Just violation immune and hormonal function of an organism causes violation common balance anabolic endergonic processes & catabolic anaerobic exergonic processes & catabolic aerobic exergonic processes of Quasi-stationary pathologic State of an organism.
Besides oncogenesis can leads as to resistance cancer cells to cytotoxic anticancer drugs as well as to relapsed cancer disease after intensive cytotoxic therapy due to following mechanisms. Just resistance cancer cells to cytotoxic anticancer drugs and relapsed cancer disease are occurred after some times of intensive chemotherapeutic treatment with large dosage cytotoxic drugs which affect nuclear DNA (nDNA) genome suppressing Meiosis-Mitosis phase cancer cellular cycle leading to apoptosis of some cancer cells death. Suppression prokaryotic genome of Meiosis-Mitosis phase cancer cellular cycle also touch on suppression both eukaryotic cells‘ DNAs genomes of Mitosis phase in immune and hormonal systems [in less ratio than cancer cells] resulting in suppression immune and hormonal systems. Moreover there are remained the retaining infected by v-oncogenes of microRNAs into destructed dead cancer cells which are ruined via expressed activity autophagy cleaning of dead cells by reticuloendothelial system, macrophages and monocytes for maintenence stability Internal Energy of an organism. Also it is occurred resistance between antiviral force of Fluoropyramidine drug and oncologic viral force for survival some viral oncogenes while suppressed both immune and hormonal systems by intensive chemotherapy with large dosage Fluoropyramidine cytotoxic drug.62,63 Thus the survived viral oncogenes with retained infected by v-oncogenes of microRNAs from destructed dead cancer can arise relapse cancer disease. It gives possibility survived some oncologic viruses [v-oncogenes] to create relapsed cancer disease after some time of intensive cytotoxic therapy. Just retaining Meiosis-Mitosis cancer cellular cycle causing by survived oncologic viruses [v-oncogenes] has also accelerating cancer cellular cycle which is occurred after restored some hormones activity after some time of intensive cytotoxic Fluoropyramidine treatment. Besides immune T memory cells, T helper cells, T killer cells with B cells are restored after some time of intensive cytotoxic activity of Fluoropyramidine treatment in which hormones restore activity of immune T memory cells, T helper cells, T killer cells. The immune T memory cells learn molecular structure of Fluoropyramidine in hormonal gland’s cells injured by Fluoropyramidine. Then T memory cells transmit the data of molecular structure Fluoropyramidine to T helper cells and further to T killer cells. Hence T killer cells destruct Fluoropyramidine causing resistance to the anticancer activity of Fluoropyramidine. However molecules of some cytotoxic drugs don’t leave data of theirs genomes in substances of injured cells of hormonal glands, and T memory cells don’t learn their molecular structures. Therefore these cytotoxic drugs are not subjected to destruction of their molecules by T killer cells. Thus there are not arisen of resistance to anticancer activity of these cytotoxic drugs.
>Sergent C. et al. studied mechanism of cytotoxic actions cisplatin and oxaliplatin in vitro, and their researches on colon cancer cells are resulted in high-level resistance of human colon cancer cells to high doses of cisplatin which does not been related to acquired defects in the DNA repaired by MMR proteins.65 Thus cytotoxic property of cisplatin acts on biosynthesis proteins via damaging translations and transcription DNA processes causing reparation by DNA mismatch repair proteins function, but cytotoxic property of cisplatin does not act on DNA reproduction for cellular proliferation. Just all necessary proteins for cells proliferations were used for experiment in vitro. Therefore survival of cancer cells in condition obtained high doses of cisplatin or oxaliplatin does not relate to acquired defects in the DNA repaired by MMR proteins corresponding to the outcomes of >Sergent C. et al. However cytotoxic property of cisplatin causes also violation processes biosynthesis immune antibodies by immune system and hormones by hormonal glands in vitro especially. However damage biosynthesis of proteins causing by cisplatin violates immune and hormonal cells’ functions although in less rate than cancer cells in vivo. Just violation immune and hormonal function of an organism causes violation common balance anabolic endergonic processes & catabolic anaerobic exergonic processes & catabolic aerobic exergonic processes of Quasi-stationary pathologic State of an organism.
Besides oncogenesis can leads as to resistance cancer cells to cytotoxic anticancer drugs as well as to relapsed cancer disease after intensive cytotoxic therapy due to following mechanisms. Just resistance cancer cells to cytotoxic anticancer drugs and relapsed cancer disease are occurred after some times of intensive chemotherapeutic treatment with large dosage cytotoxic drugs which affect nuclear DNA (nDNA) genome suppressing Meiosis-Mitosis phase cancer cellular cycle leading to apoptosis of some cancer cells death. Suppression prokaryotic genome of Meiosis-Mitosis phase cancer cellular cycle also touch on suppression both eukaryotic cells‘ DNAs genomes of Mitosis phase in immune and hormonal systems [in less ratio than cancer cells] resulting in suppression immune and hormonal systems. Moreover there are remained the retaining infected by v-oncogenes of microRNAs into destructed dead cancer cells which are ruined via expressed activity autophagy cleaning of dead cells by reticuloendothelial system, macrophages and monocytes for maintenence stability Internal Energy of an organism. Also it is occurred resistance between antiviral force of cisplatin or oxaliplatin drugs and oncologic viral force for survival some viral oncogenes while suppressed both immune and hormonal systems by intensive chemotherapy with large dosage cisplatin or oxaliplatin cytotoxic drug.62,63 Thus the survived viral oncogenes with retained infected by v-oncogenes of microRNAs from destructed dead cancer can arise relapse cancer disease. It gives possibility survived some oncologic viruses [v-oncogenes] to create relapsed cancer disease after some time of intensive cytotoxic therapy. Just retaining Meiosis-Mitosis cancer cellular cycle causing by survived oncologic viruses [v-oncogenes] has also accelerating cancer cellular cycle which is occurred after restored some hormones activity after some time of intensive cytotoxic cisplatin or oxaliplatin treatment. Besides immune T memory cells, T helper cells, T killer cells with B cells are restored after some time of intensive cytotoxic activity of cisplatin or oxaliplatin treatment in which hormones restore activity of immune T memory cells, T helper cells, T killer cells. The immune T memory cells learn molecular structure of cisplatin or oxaliplatin in hormonal gland’s cells injured by cisplatin or ovaliplatin. Then T memory cells transmit the data of molecular structure cisplatin or oxaliplatin to T helper cells and further to T killer cells. Hence T killer cells destruct cisplatin or oxaliplatin causing resistance to the anticancer activity of cisplatin or oxaliplatin.
However molecules of some cytotoxic drugs don’t leave data of theirs genomes in substances of injured cells of hormonal glands, and T memory cells don’t learn their molecular structures. Therefore these cytotoxic drugs are not subjected to destruction of their molecules by T killer cells. Thus there are not arisen of resistance to anticancer activity of these cytotoxic drugs. Just this situation can be arisen in experiment in vitro. >Stubbert LJ. et al. studied mechanism of resistance to cytotoxic action cisplatin in cancer cells.66 Their researches on several prostate cancer cells and colorectal carcinoma cells are resulted in following data. Cisplatin resistance is multi-factorial process but can be associated with DNA repair capacity either mutations with p53 or loss DNA mismatch repair capacity. However these processes don’t lead to resistance to cytotoxic action cisplatin in cancer cells without operations immune defensive mechanisms. Indeed cytotoxic actions of cisplatin on biosynthesis proteins results in decreased transcription-coupled nucleotide leading to damaging translations in DNA biosynthesis proteins processes as in cancer cells as well as in an organism’s immune and hormonal cells. Thus citotoxic property cisplatin causes violation processes biosynthesis immune antibodies by immune system and hormones by hormonal glands. Hence damage biosynthesis of proteins causing by cisplatin violates immune and hormonal cells’ functions although in less rate than cancer cells. Just violation immune and hormonal function of an organism causes violation common balance anabolic endergonic processes & catabolic anaerobic exergonic processes & catabolic aerobic exergonic processes of Quasi-stationary pathologic State of an organism.
Besides oncogenesis can leads as to resistance cancer cells to cytotoxic anticancer drugs as well as to relapsed cancer disease after intensive cytotoxic therapy due to following mechanisms. Just resistance cancer cells to cytotoxic anticancer drugs and relapsed cancer disease are occurred after some times of intensive chemotherapeutic treatment with large dosage cytotoxic drugs which affect nuclear DNA (nDNA) genome suppressing Meiosis-Mitosis phase cancer cellular cycle leading to apoptosis of some cancer cells death. Suppression prokaryotic genome of Meiosis-Mitosis phase cancer cellular cycle also touch on suppression both eukaryotic cells‘ DNAs genomes of Mitosis phase in immune and hormonal systems [in less ratio than cancer cells] resulting in suppression immune and hormonal systems. Moreover there are remained the retaining infected by v-oncogenes of microRNAs into destructed dead cancer cells which are ruined via expressed activity autophagy cleaning of dead cells by reticuloendothelial system, macrophages and monocytes for maintenence stability Internal Energy of an organism. Also it is occurred resistance between antiviral force of cisplatin drug and oncologic viral force for survival some viral oncogenes while suppressed both immune and hormonal systems by intensive chemotherapy with large dosage cisplatin cytotoxic drug.62,63 Thus the survived viral oncogenes with retained infected by v-oncogenes of microRNAs from destructed dead cancer can arise relapse cancer disease. It gives possibility survived some oncologic viruses [v-oncogenes] to create relapsed cancer disease after some time of intensive cytotoxic therapy. Just retaining Meiosis-Mitosis cancer cellular cycle causing by survived oncologic viruses [v-oncogenes] has also accelerating cancer cellular cycle which is occurred after restored some hormones activity after some time of intensive cytotoxic cisplatin treatment. Besides immune T memory cells, T helper cells, T killer cells with B cells are restored after some time of intensive cytotoxic activity of cisplatin treatment in which hormones restore activity of immune T memory cells, T helper cells, T killer cells. The immune T memory cells learn molecular structure of cisplatin in hormonal gland’s cells injured by cisplatin. Then T memory cells transmit the data of molecular structure cisplatin to T helper cells and further to T killer cells. Hence T killer cells destruct cisplatin causing resistance to the anticancer activity of cisplatin.
However molecules of some cytotoxic drugs don’t leave data of theirs genomes in substances of injured cells of hormonal glands, and T memory cells don’t learn their molecular structures. Therefore these cytotoxic drugs are not subjected to destruction of their molecules by T killer cells. Thus there are not arisen of resistance to anticancer activity of these cytotoxic drugs. Lin X. and Howell S.B. examined influences loss of p53 or DNA mismatch repair proteins (MMR) function on resistance to cisplatin, i.e. they examined mechanism resistance to cisplatin in loss of p53 or DNA mismatch repair (MMR) function.67 Just DNA mismatch repair proteins and p53 function are major determinants of the rate cisplatin resistance concerning to the outcomes of their researches. Also Lin X. and Howell S.B. proclaim “As opposed to factors that control sensitivity to the cytotoxic effect of cisplatin, little is known about the factors that determine the reaction which resistance develops”. Thus Lin X. and Howell S.B. made conclusion that separate from effects on sensitivity to the cytotoxic effect of cisplatin, loss of MMR, especially when it combined with loss of p53, results in rapid evolution of cisplatin resistance during sequential rounds of drug exposure that is likely mediated by enhanced mutagenic translation synthesis. The DNA damage response activated by cisplatin is accompanied by a p53- and MMR-dependent increase in homologous recombination even between adduct of free sequences. Further Lin X. et al. studied P53 modulates the effect of loss of DNA mismatch repair on the sensitivity of human colon cancer cells to the cytotoxic and mutagenic effects of cisplatin.67 Their experiments are shown that disruption of p53 in MMR-deficient HCT116 cells resulted in substantial levels of resistance to some agents (paclitaxel, 1.9-fold; gemcitabine, 2.7-fold; 6-thioguanine, 3.3-fold; and etoposide, 4.4-fold) but low sensitization to other agents (topotecan, 2.5-fold; and DDP, 3.3-fold). Loss of MMR or p53 alone had only minor effect on sensitivity to the mutagenic effect of DDP as measured by the appearance of variants resistant to 6-thioguanine, etoposide, topotecan, gemcitabine, and paclitaxel in the population 10 days later (1.0-2.4-fold), whereas loss of both p53 and MMR had a more profound effect (1.7-6.5-fold). Loss of both p53 and MMR increased the basal frequency insertion/deletion mutations detected by a shuttle vector-based assay to a greater extent than loss of either alone. The Lin X. et al. note that these results indicate that p53 and MMR can cooperate to control sensitivity to the cytotoxic effect of DDP and to limit its mutagenic potential in the colon cancer cells.
However Lin X. et al. researches don’t determine mechanism of resistance to cisplatin and other cytotoxic drugs, but they determine results of their negative actions which happened simultaneously with resistance to cytotoxic action of these drugs. Also researches LinX et all expose both mutual interaction between relative mechanisms p53 cancer suppressor binding chromosome 17, DNA Mismatch Repair Protein (MMR) acting as in interphase S / G2 as well as on chromosome 3 in Mitosis (M) phase and resistance to cytotoxic drugs by cancer cells, i.e. not separating either interactions on driving mechanism cellular cycle which advances due to interactions as between nuclear center how mechanism of anabolic processes and mitochondrial center how catabolic processes of defensive mechanisms in inner cells or resistance to cisplatin influences on cancer cells by defensive mechanisms of immune mechanism of an organism in outer cells medium. These interactions are realized by alternations inflow and outflow substances and energy forming prevalence intracellular chemical potential (µinner cell) or extracellular chemical potential (µouter cell) [µinner cell >↔˂ µouter cell] corresponding to Theorell equation which realize driving mechanism as cancer cellular cycle for cancer development as well as immune and hormonal cellular cycles for these cells activities reflecting positive fluctuations (+Δxβ) and negative fluctuation entropy (-Δxβ) advancing cellular cycle through G0/G1/S/G2/M phases according Glansdorff-Prigogine theory(Figure 3).63,69 Therefore influences both p53 and MMR [loss or activity, deficient or proficient] are genetic mechanisms of interactions between violations of some parts of DNA and Mismatch Repair Protein (MMR) with p53 cellular suppressor via binding chromasomes 17, 3 etc in Mitosis or Meiosis-Mitosis phases of cellular cycle in immune cells and hormonal cells or cancer cells. But resistance to different cytotoxic drugs depend as on cancer cells reaction against cytotoxic activity of drug via rearranged cellular cycle for cancer cells survival as well as on immune cells reactions on substances of cytotoxic drug corresponding to Schreodinger method of the molecular orbitals – a linear combination of atomic orbitals (LCAO-MO).
Thus loss of p53 or DNA mismatchrepair (MMR) function leads to violation of activity cancer cells only a little because accelerated proliferation with forming metastases of prokaryotic cancer cellular cycle does not need repairing function. As concerning to cells of an organism, loss of p53 or DNA mismatchrepair (MMR) function require reparation especially immune Tcells, immune B cells, macrophages, monocytes and the other blood cells engaged in immune defensive function of an organism and in autophagy of cleaning from dead cells of an organism‘s Internal Energy versus the all other cells of an organism. However cisplatin drug damages anabolic mechnism of biosynthesis proteins of cancer cells and causing suppression anabolic mechnism of biosynthesis proteins of immune and hormonal cells. Therefore the immune and hormonal cells must be restored after actions of large dosage cytotoxic cisplatin drug for the activities as immune defensive function of immune Tcells, immune B cells as well as function of macrophages, monocytes and the other blood cells engaged in autophagy of cleaning from dead cells of an organism‘s Internal Energy versus the other cells of an organism. The suppression organism’s cells including immune and hormonal cells after actions of cytotoxic cisplatin drug are restored by cells themseves without activities of p53 or DNA mismatchrepair (MMR) function. Thus target of chemotherapeutic treatment with cisplatin and other cytotoxic drugs affect nuclear DNA (nDNA) suppressing as G1/S/G2 phases an organism’s cells’ cellular cycle as well as Meiosis-Mitosis phase cancer cellular cycle which touch on as human eukaryotic genome as well as oncologic prokaryotic genome. It is occurred resistance between antiviral force of cisplatin or of other cytotooxic drugs and oncologic viral [v-oncogene] force for survival viral oncogenes activities in which transition Meiosis-Mitosis into Mitosis-Meiosis reflects prevalence antiviral force of cisplatin. However damage biosynthesis of proteins causing by cisplatin violates immune and hormonal cells’ functions although in less rate than cancer cells. Just violation immune and hormonal function of an organism causes violation common balance anabolic endergonic processes & catabolic anaerobic exergonic processes & catabolic aerobic exergonic processes of Quasi-stationary pathologic State of an organism. Besides oncogenesis can leads as to resistance cancer cells to cytotoxic anticancer drugs as well as to relapsed cancer disease after intensive cytotoxic therapy due to following mechanisms. Just resistance cancer cells to cytotoxic anticancer drugs and relapsed cancer disease are occurred after some times of intensive chemotherapeutic treatment with large dosage cytotoxic drugs which affect nuclear DNA (nDNA) genome suppressing Meiosis-Mitosis phase cancer cellular cycle leading to apoptosis of some cancer cells death. Suppression prokaryotic genome of Meiosis-Mitosis phase cancer cellular cycle also touch on suppression both eukaryotic cells‘ DNAs genomes of Mitosis phase in immune and hormonal systems [in less ratio than cancer cells] resulting in suppression immune and hormonal systems. Moreover there are remained the retaining infected by v-oncogenes of microRNAs into destructed dead cancer cells which are ruined via expressed activity autophagy cleaning of dead cells by reticuloendothelial system, macrophages and monocytes for maintenence stability Internal Energy of an organism. Also it is occurred resistance between antiviral force of cisplatin drug and oncologic viral force for survival some viral oncogenes while suppressed both immune and hormonal systems by intensive chemotherapy with large dosage cisplatin cytotoxic drug.62,63 Thus the survived viral oncogenes with retained infected by v-oncogenes of microRNAs from destructed dead cancer can arise relapse cancer disease. It gives possibility survived some oncologic viruses [v-oncogenes] to create relapsed cancer disease after some time of intensive cytotoxic therapy. Just retaining Meiosis-Mitosis cancer cellular cycle causing by survived oncologic viruses [v-oncogenes] has also accelerating cancer cellular cycle which is occurred after restored some hormones activity after some time of intensive cytotoxic cisplatin treatment. Besides immune T memory cells, T helper cells, T killer cells with B cells are restored after some time of intensive cytotoxic activity of cisplatin treatment in which hormones restore activity of immune T memory cells, T helper cells, T killer cells. The immune T memory cells learn molecular structure of cisplatin in hormonal gland’s cells injured by cisplatin. Then T memory cells transmit the data of molecular structure cisplatin to T helper cells and further to T killer cells. Hence T killer cells destruct cisplatin causing resistance to the anticancer activity of cisplatin.
However molecules of some cytotoxic drugs don’t leave data of theirs genomes in substances of injured cells of hormonal glands, and T memory cells don’t learn their molecular structures. Therefore these cytotoxic drugs are not subjected to destruction of their molecules by T killer cells. Thus there are not arisen of resistance to anticancer activity of these cytotoxic drugs. Nehmé A. et al. studied inducing of JNK and c-Abl signalling by cisplatin and oxaliplatin in mismatch repair-proficient and -deficient cells causing resistance to theses cytotoxic drugs.69 Their studies have shown that loss of DNA mismatch repair results in resistance to cisplatin but not to oxaliplatin, supposing that the mismatch repair proteins serve as a detector for cisplatin but not oxaliplatin adduct. The identifying the transduction pathways with the detector communicates, they investigated the effect of loss DNA mismatch repair on activation of known damage-responsive pathways, and recently reported that cisplatin differetially activates c-Jun NH2-terminal kinase (JNK) and c-Abl in repair-proficient vs.-deficient cells.
Nehme A. et al. don’t determine chemotherapeutic targets of cancer cells which were chosen for cytotoxic treatment by cisplatin and oxaliplatin. As concerning inducing of JNK and c-Abl signalling by cisplatin and oxaliplatin in mismatch repair-proficient and -deficient cells, this inducing does not influence on mechanism of restoring cells of immune and hormonal systems after actions large dosage of cytotoxic cisplatin and oxaliplatin. Just target of chemotherapeutic treatment with cisplatin and oxaliplatin affect nuclear DNA (nDNA) suppressing as G1/S/G2 phases cancer cellular cycle as well as Meiosis-Mitosis phase cancer cellular cycle which touch on as oncologic viral [v-oncogene] prokaryotic genome as well as human eukaryotic genome of cells both immune system and hormonal system. It is occurred resistance between antiviral force of cisplatin or oxaliplatin and oncologic viral [v-oncogene] force for survival in which transition Meiosis-Mitosis into Mitosis-Meiosis reflects prevalence antiviral force of cisplatin.
Besides oncogenesis can leads as to resistance cancer cells to cytotoxic anticancer drugs as well as to relapsed cancer disease after intensive cytotoxic therapy due to following mechanisms. Just resistance cancer cells to cytotoxic anticancer drugs and relapsed cancer disease are occurred after some times of intensive chemotherapeutic treatment with large dosage cytotoxic drugs which affect nuclear DNA (nDNA) genome suppressing Meiosis-Mitosis phase cancer cellular cycle leading to apoptosis of some cancer cells death. Suppression prokaryotic genome of Meiosis-Mitosis phase cancer cellular cycle also touch on suppression both eukaryotic cells‘ DNAs genomes of Mitosis phase in immune and hormonal systems [in less ratio than cancer cells] resulting in suppression immune and hormonal systems. Moreover there are remained the retaining infected by v-oncogenes of microRNAs into destructed dead cancer cells which are ruined via expressed activity autophagy cleaning of dead cells by reticuloendothelial system, macrophages and monocytes for maintenence stability Internal Energy of an organism. Also it is occurred resistance between antiviral force of cisplatin or oxaliplatin drugs and oncologic viral force for survival some viral oncogenes while suppressed both immune and hormonal systems by intensive chemotherapy with large dosage cisplatin or oxaliplatin cytotoxic drug.62,63 Thus the survived viral oncogenes with retained infected by v-oncogenes of microRNAs from destructed dead cancer can arise relapse cancer disease. It gives possibility survived some oncologic viruses [v-oncogenes] to create relapsed cancer disease after some time of intensive cytotoxic therapy. Just retaining Meiosis-Mitosis cancer cellular cycle causing by survived oncologic viruses [v-oncogenes] has also accelerating cancer cellular cycle which is occurred after restored some hormones activity after some time of intensive cytotoxic cisplatin or oxaliplatin treatment. Besides immune T memory cells, T helper cells, T killer cells with B cells are restored after some time of intensive cytotoxic activity of cisplatin or oxaliplatin treatment in which hormones restore activity of immune T memory cells, T helper cells, T killer cells. The immune T memory cells learn molecular structure of cisplatin or oxaliplatin in hormonal gland’s cells injured by cisplatin or ovaliplatin. Then T memory cells transmit the data of molecular structure cisplatin or oxaliplatin to T helper cells and further to T killer cells. Hence T killer cells destruct cisplatin or oxaliplatin causing resistance to the anticancer activity of cisplatin or oxaliplatin.
However molecules of some cytotoxic drugs don’t leave data of theirs genomes in substances of injured cells of hormonal glands, and T memory cells don’t learn their molecular structures. Therefore these cytotoxic drugs are not subjected to destruction of their molecules by T killer cells. Thus there are not arisen of resistance to anticancer activity of these cytotoxic drugs. Fink D, et al. studied role of DNA mismatch repair in platinum drug resistance.70 Loss of DNA mismatch repair occurs in many types of tumors. The effect of the loss of DNA mismatch repair activity on sensitivity to cisplatin and a panel of analogues drugs were tested using two pairs of the DNA mismatch repair proteins in cell lines proficient or deficient of this function. HCT116+ch2, a human colon cancer cell line deficient in hMLH1, was 2.1-fold resistant to cisplatin and 1.3-fold resistant to carboplatin when compared to a subline complemented with chromosome 3 expressing a wild-type copy of hMLH1. Likewise, the human endometrial cancer cell line HEC59, which is deficient in hMSH2, was 1.8-fold resistant to cisplatin and 1.5-fold resistant to carboplatin when compared to a subline complemented with chromosome 2 with a wild-type hMSH2. In contrast to cisplatin and carboplatin, which form the same types of adducts in DNA, there was no difference in sensitivity between the DNA mismatch repair-proficient and -deficient cell lines for oxaliplatin, tetraplatin, transplatin, JM335, or JM216. The formation of protein-DNA complexes that contained hMSH2 and hMLH1 was documented by mobility shift assay when nuclear extracts were incubated with DNA platinated with cisplatin but not with oxaliplatin. These results demonstrate a correlation between failure of the DNA mismatch repair proteins to recognize the platinum adduct and low-level resistance, suggesting a role the DNA mismatch repair system in generating signals that contribute to the generation of apoptotic activity. They also identify the use of drugs whose adducts are not recognized as a strategy for circumventing resistance due to loss of DNA mismatch repair.
Thus Fink D. et al. don’t determine chemotherapeutic targets of cancer cells which were chosen for cytotoxic treatment by platinum drugs resistance.70 Besides studying influences DNA mismatch repair proficient and deficient lines on different platinum cytotoxic drugs resistance, Fink D. et al. notes following „These results demonstrate a correlation between failure of the DNA mismatch repair proteins to recognize the platinum adduct and low-level resistance, suggesting a role the DNA mismatch repair system in generating signals that contribute to the generation of apoptotic activity“. It’s meant that DNA mismmatch repair function is not carried out showing only law-level resistance to cytotoxic drugs [or maybe no resistance?]. Hence target of chemotherapeutic treatment with cisplatin and other platinum drugs affect nuclear DNA (nDNA) suppressing as G1/S/G2 phases cancer cellular cycle as well as Meiosis-Mitosis phase cancer cellular cycle which touch on as oncologic viral oncogene [v-oncogene] prokaryotic genome as well as human eukaryotic genome of all cells. It is occurred resistance between antiviral force of cisplatin or other platinum drugs and oncologic viral [v-oncogene] force for survival in which transition Meiosis-Mitosis into Mitosis-Meiosis reflects prevalence antiviral force of cisplatin or other platinum drugs.
Besides oncogenesis can leads as to resistance cancer cells to cytotoxic anticancer drugs as well as to relapsed cancer disease after intensive cytotoxic therapy due to following mechanisms. Just resistance cancer cells to cytotoxic anticancer drugs and relapsed cancer disease are occurred after some times of intensive chemotherapeutic treatment with large dosage cytotoxic drugs which affect nuclear DNA (nDNA) genome suppressing Meiosis-Mitosis phase cancer cellular cycle leading to apoptosis of some cancer cells death. Suppression prokaryotic genome of Meiosis-Mitosis phase cancer cellular cycle also touch on suppression both eukaryotic cells‘ DNAs genomes of Mitosis phase in immune and hormonal systems [in less ratio than cancer cells] resulting in suppression immune and hormonal systems. Moreover there are remained the retaining infected by v-oncogenes of microRNAs into destructed dead cancer cells which are ruined via expressed activity autophagy cleaning of dead cells by reticuloendothelial system, macrophages and monocytes for maintenence stability Internal Energy of an organism. Also it is occurred resistance between antiviral force of cisplatin or other platinums drugs and oncologic viral force for survival some viral oncogenes while suppressed both immune and hormonal systems by intensive chemotherapy with large dosage cisplatin or other platinums cytotoxic drug.62,63 Thus the survived viral oncogenes with retained infected by v-oncogenes of microRNAs from destructed dead cancer can arise relapse cancer disease. It gives possibility survived some oncologic viruses [v-oncogenes] to create relapsed cancer disease after some time of intensive cytotoxic therapy. Just retaining Meiosis-Mitosis cancer cellular cycle causing by survived oncologic viruses [v-oncogenes] has also accelerating cancer cellular cycle which is occurred after restored some hormones activity after some time of intensive cytotoxic cisplatin or other platinums treatment. Besides immune T memory cells, T helper cells, T killer cells with B cells are restored after some time of intensive cytotoxic activity of cisplatin or other platinums treatment in which hormones restore activity of immune T memory cells, T helper cells, T killer cells. The immune T memory cells learn molecular structure of cisplatin or other platinums in hormonal gland’s cells injured by cisplatin or other platinums. Then T memory cells transmit the data of molecular structure cisplatin or other platinums to T helper cells and further to T killer cells. Hence T killer cells destruct cisplatin or other platinums causing resistance to the anticancer activity of cisplatin or other platinums.
However molecules of some cytotoxic drugs don’t leave data of theirs genomes in substances of injured cells of hormonal glands, and T memory cells don’t learn their molecular structures. Therefore these cytotoxic drugs are not subjected to destruction of their molecules by T killer cells. Thus there are not arisen of resistance to anticancer activity of these cytotoxic drugs. Yang Zhang et al. researches resulted in tumor regrowth after chemotherapy with fluorouracil which suppress immune system.71 Authors have proclaimed conclusion, that damage immune system leads to tumor regrowth. Luo J. et al. have also proclaimed conclusion that cancer tumor leads to regrowth and creates metastasis in spite of being over chemotherapy.72 O Connell MJ. et al. studied patients with colon cancer of stage II/III after treatment by surgery alone or surgery plus chemotherapy fluorouracil plus leucovorin73 and received results that tumor was removed in first event and recurrence cancer in second events. Kast R.E. et al. studied treatment approach with nine cytotoxic drugs for research of relapsed Glioblastoma74 and received accelerated growth of Glioblastoma. Gifford J.B. et al. studied treatment Pancreatic ductal Adenocarcinoma and result in expression GRP78 protein which appearance characterizes induced resistance chemotherapeutic treatment which leads to relapsed Adenocarcinoma.75
Thus researches Yang Zhang et al., Luo J. et al., O. Connell M.J. et al., Kast R.E. et al., Gifford J.B. et al. have not considered as targets of cytotoxic drugs operations on viral oncogene [v-oncogene] which driving mechanisms cause as relapsed cancer disease as well as interactions between oncologic viruses, hormonal system and immune system of an organism in processes resistance to cytotoxic drugs and process of relapsed cancer disease. Just target of chemotherapeutic treatment with large dosage cytotoxic drugs affect nuclear DNA (nDNA) suppressing as G1/S/G2 phases cancer cellular cycle as well as Meiosis-Mitosis phase cancer cellular cycle which touch on as oncologic viral [v-oncogene] prokaryotic genome as well as human eukaryotic genome of all cells of an organism including cells of immune and hormonal systems. It is occurred resistance between antiviral force of cytotoxic drugs and viral oncogene [v-oncogene] force for survival in which transition Meiosis-Mitosis into Mitosis-Meiosis reflects prevalence antiviral forse. Just oncologic viruses [v-oncogenes] use rest hormonal mechanisms (not damaged by cytotoxic drugs) for supporting cancer cells development via accelerating cellular cycle.62,63
Besides oncogenesis can leads as to resistance cancer cells to cytotoxic anticancer drugs as well as to relapsed cancer disease after intensive cytotoxic therapy due to following mechanisms. Just resistance cancer cells to cytotoxic anticancer drugs and relapsed cancer disease are occurred after some times of intensive chemotherapeutic treatment with large dosage cytotoxic drugs which affect nuclear DNA (nDNA) genome suppressing Meiosis-Mitosis phase cancer cellular cycle leading to apoptosis of some cancer cells death. Suppression prokaryotic genome of Meiosis-Mitosis phase cancer cellular cycle also touch on suppression both eukaryotic cells‘ DNAs genomes of Mitosis phase in immune and hormonal systems [in less ratio than cancer cells] resulting in suppression immune and hormonal systems. Moreover there are remained the retaining infected by v-oncogenes of microRNAs into destructed dead cancer cells which are ruined via expressed activity autophagy cleaning of dead cells by reticuloendothelial system, macrophages and monocytes for maintenence stability Internal Energy of an organism. Also it is occurred resistance between antiviral force of cytotoxic drug and oncologic viral force for survival some viral oncogenes while suppressed both immune and hormonal systems by intensive chemotherapy with large dosage cytotoxic drug.62,63 Thus the survived viral oncogenes with retained infected by v-oncogenes of microRNAs from destructed dead cancer can arise relapse cancer disease. It gives possibility survived some oncologic viruses [v-oncogenes] to create relapsed cancer disease after some time of intensive cytotoxic therapy. Just retaining Meiosis-Mitosis cancer cellular cycle causing by survived oncologic viruses [v-oncogenes] has also accelerating cancer cellular cycle which is occurred after restored some hormones activity after some time of intensive cytotoxic drug treatment. Besides immune T memory cells, T helper cells, T killer cells with B cells are restored after some time of intensive cytotoxic activity of cytotopxic drug treatment in which hormones restore activity of immune T memory cells, T helper cells, T killer cells. The immune T memory cells learn molecular structure of the cytotoxic drug in hormonal gland’s cells injured by this cytotoxic drug. Then T memory cells transmit the data of molecular structure the cytotoxic drug to T helper cells and further to T killer cells. Hence T killer cells destruct this cytotoxic drug causing resistance to the anticancer activity of the cytotoxic drug. However molecules of some cytotoxic drugs don’t leave data of theirs genomes in substances of injured cells of hormonal glands, and T memory cells don’t learn their molecular structures. Therefore these cytotoxic drugs are not subjected to destruction of their molecules by T killer cells. Thus there are not arisen of resistance to anticancer activity of these cytotoxic drugs.
Discussion exerted organism‘s mechanisms sensitivity causing resistance to some cytotoxic anticancer drugs as well as relapsed cancer disease after chemotherapy with large dosage of cytotoxic drags
As concerning to recurrence cancer disease after intensive chemotherapy with large dosages of cytotoxic drugs, the mechanisms recurrence cancer disease was described by authors in following modes: The some authors noted that suppression of cells immune and hormonal systems by large dosages of cytotoxic drugs cause recurrence cancer disease after cytotoxic chemotherapy. The other authors noted that unsufficient or unefficient chemotherapy leads to relapsed cancer disease. However it must be considered the interactions between restoring of depressed oncologic virises [v-oncogenes], restoring of some depressed immune system and restoring of some depressed hormonal system after intensive chemotherapy with large doses of cytotoxic drugs.56,57,59, 60,63,64 The intensive chemotherapy with large dosage cytotoxic drugs suppress as cancer cellular cycle as well as immune system mechanisms and hormonal system mechanism causing some violation maintenance stability Internal Energy of an organism.76–78 Just the target of chemotherapeutic treatment with cytotoxic drugs is nuclear DNA (nDNA) that causes suppression as G1/S/G2 phases cancer cellular cycle as well as Meiosis-Mitosis phase cancer cellular cycle which touch on as viral prokaryotic genome as well as human eukaryotic genome. After some time cessation of chemotherapy with large dosage cytotoxic drugs or some weakening chemotherapy, it is occurred restoring suppressed both hormonal glands activity via production hormones and immune cells which is stimulated by cellular capacitors operation of an organism’s both immune cells and hormonal cells. Just especially immune cells of T lymphocytes and B lymphocytes operations result in gradual recovery Internal Energy of an organism. Simultaneously it is occurred restoring cells cellular capacitors, affected by viral oncogenes [v-oncogenes]. Further it is happened resistance between antiviral force of cytotoxic drugs and viral oncogenes [v-oncogene] force for their survival in which transition Meiosis-Mitosis into Mitosis-Meiosis reflects prevalence antiviral force of cytotoxic drugs. But retaining Meiosis-Mitosis reflects prevalence viral force for viral survival in which oncologic viruses [v-oncogenes] use rest hormonal mechanisms (not damaged by cisplatin) for supporting cancer cells development via accelerating cellular cycle.63,64 Just retaining Meiosis-Mitosis cancer cellular cycle exerts accelerating cancer cellular cycle causing survived oncologic viruses [v-oncogenes], which is arisen by remained data of infected microRNAs from destructed dead cancer cells because of affected by cytotoxic drugs. Besides restored hormonal mechanisms of hormones activity after some time of intensive cytotoxic drug treatment accepted molecular structure of cytotoxic drug injured her. It gives possibility to learn data of molecular structure of cytotoxic drug in restored hormonal glands with hormones for some survived oncologic viruses [v-oncogenes] which create relapsed cancer disease after some time of intensive cytotoxic therapy. Just rest hormonal glands exert hormones activity and immune T memory cells, T helper cells, T killer cells with B cells which are restored after some time of intensive cytotoxic drugs treatment in which hormones restore activity of immune T memory cells, T helper cells, T killer cells. The resonance waves of cellular capacitors of immune T cells memory learn molecule of cytotoxic drug as strange substance into hormonal gland’s cells injured by this cytotoxic drug. Then the data of the cytotoxic drug molecule is transmited from T memory cells to T helper cells, and further the data of the cytotoxic drug molecule is transmited from T helper cells to T killer cells. The T killer cells destruct the molecule of cytotoxic drug causing by enzymes of their lysosomes due to resonance waves of their capacitors operation corresponing to the resonance wave functions on molecular orbital of cytotoxic drugs’ substances corresponding to the method molecular orbitals – a linear combination of atomic orbitals (MO LCAO), according outstanding Schreodinger equaton (Figure 4).59 Thus there are occurred the reaction resonance waves on the cytotoxic drugs as the strange substances that cause attraction cytotoxic drug as Ligant to the variable capacitors of the Receptors on the walls of immune Tkiller cells59 (Figure 4). It forms link Receptor-Ligant. Then it is occurred internalization Receptor-Ligant into immune Tkiller cells due to resonance waves of cellular capacitors operation. The T killer cells, being sensitized by T helper via their resonance waves of cellular capacitors, destroy drugs’ substances promoting attraction Lisosomes to the drugs for decomposing drug substances by lisosomal enzymes. Thus immune system of T cells realize resistance to cytotoxic drugs by the immune reaction of sensibilized immune T cells of an organism via link T memory cells → T helper cells → T killer cells causing reaction on strange substances of wave function of cytotoxic drugs’ molecule orbits resulting in resistance to the cytotoxic drug influences. Thus it is happened the resistance to the anticancer activity of cytotoxic drug. On the other hand, some molecules of cytotoxic drugs don’t leave data of theirs genomes in substances of injured cells of hormonal glands, and T memory cells don’t learn structure of their molecules. Therefore these cytotoxic drugs are not subjected to destruction of their molecules by T killer cells. Thus there are not arisen of resistance to anticancer activity of these cytotoxic drugs.
Considering mechanisms affecting by viral oncogenes (v-oncogenes) of deep infected Pluripotent stem cells (iPSCs) as well as Oligopotent stem cells and Unipotent stem cells of cellular genomes, the activities of defensive mechanisms of both immune and hormonal systems of an organism are also suppressed by the viral oncogenes showing more strong viral oncogene over organism cells‘ genomes.3–16 Cancer cells‘ proliferations form cancer tumor which is situated inside the human organism using the organism as Environment, and obtains the substances from depot of an organism (fat depots, carbohydrate depots etc.) for its metabolism. The organism obtains substances also from depots of an organism for its metabolism in condition of new method cancer treatment by “Prolonged medical Starvation (during 42-45 days)”.78–81 The treatment by “Prolonged medical Starvation (during 42-45 days)” causes considerable decreased almost of all depots of an organism especially exhausting organism’s fat and hydrocarbonic depots, that leads to competition between cancer tissue and normal tissue of an organism for the use of remained decreased depot needing for metabolism of cancer cells and for maintenance stability Internal Energy of an organism and its cells (Uorg) (normal temperature 36,0ºC-37,0°C by which all enzymes operate and other indices). Thus this competition between the organism and the cancer cells must lead to the win for most strong one. But the protective forces of the organism become stronger due to support of an organism with herbal extracts, delivering vitamins and microelements into the organism during “Prolonged medical Starvation (during 42-45 days)” that suppress excessive Anabolic biosynthesis of cancer tissue and cancer cells (Figure 5).78–81 Besides increase of fat metabolism from fat depot leads to augmentation glutathione peroxide (GPX) and phospholipid hydroperoxide glutathione peroxidise (PHGPX) in all cells of an organism which neutralize redundant superoxide [O*] and ROS/H2O2/free radicals in G1/S phases cellular cycle of cancer cells cycle suppressing excessive proliferative processes of cancer cells.26,27 Suppression accelerating cellular cycle in cancer cellular cycle because of decreased anabolic processes in condition of “Prolonged medical Starvation (during 42-45 days)” exert normal nuclear DNA [nDNA] replication via prevailing Mitosis over Meiosis in combined cancer cellular cycle’s Mitosis-Meiosis phase of nuclear DNA [nDNA].20–22 Eliminating partial suppression of Anaerobic processes of oxidative phosphorilation by “Prolonged medical Starvation (during 42-45 days)” restores normal balance Catabolic aerobic oxidative processes & Catabolic anaerobic processes of oxidative phosphorilation in mitochondria of cancer cells decreasing Reactive Oxygen Species (ROS) in mitochondria of cancer cells (Figure 5).26,27 causing suppression of excessive nucleus DNA replication with normalization of cellular cycle of cancer cells and elimination irrepressible proliferative processes of cancer growth. Also complex Mitosis-Meiosis phase of cancer cellular cycle is partial broken into prevailing separate diploid Mitosis phase where haploid Meiosis phase of viral cellular cycle is deprived for exerting normal cellular cycle. Besides partial broken covalent bonds between Mitosis and Meiosis deprive barriering defence of viral pluripotent stem cells function causing normal cellular cycle with activity of diploid Mitosis phase in cancer cells.23 Thus the power of an organism’s energy become stronger than viral oncogene (v-oncogene) energy, and cancer cells’ metabolism is plunged into depression. Then the very small quantity of weak cytotoxic substances destruct depressed cancer cells resulting in their death by new method cancer treatment. The increased quantity microRNAs from ruined dead cells stimulate prevail activity of proteins CTLA-4, PD-1 in interactions between proteins CTLA-4, PD-1 and CD-28, CD-80 (B-7-1), CD-86 (B-7-2) in an organism that exert as autophagy of increased macrophages and monocytes as well as immune activity of Tcells. Thus the new method cancer treatment use also mechanisms as of both immune system and hormonal system for destruction dead cancer cells by immune defensive processes of phagocytosis [T memory cells → T helper cells → T killer cells causing destruction dead cells] as well as processes cleaning Internal Energy (U) of an organism by autophagy of macrophages and monocytes for maintenance stability Internal Energy (U) in an organism in norm and in pathology.78–82
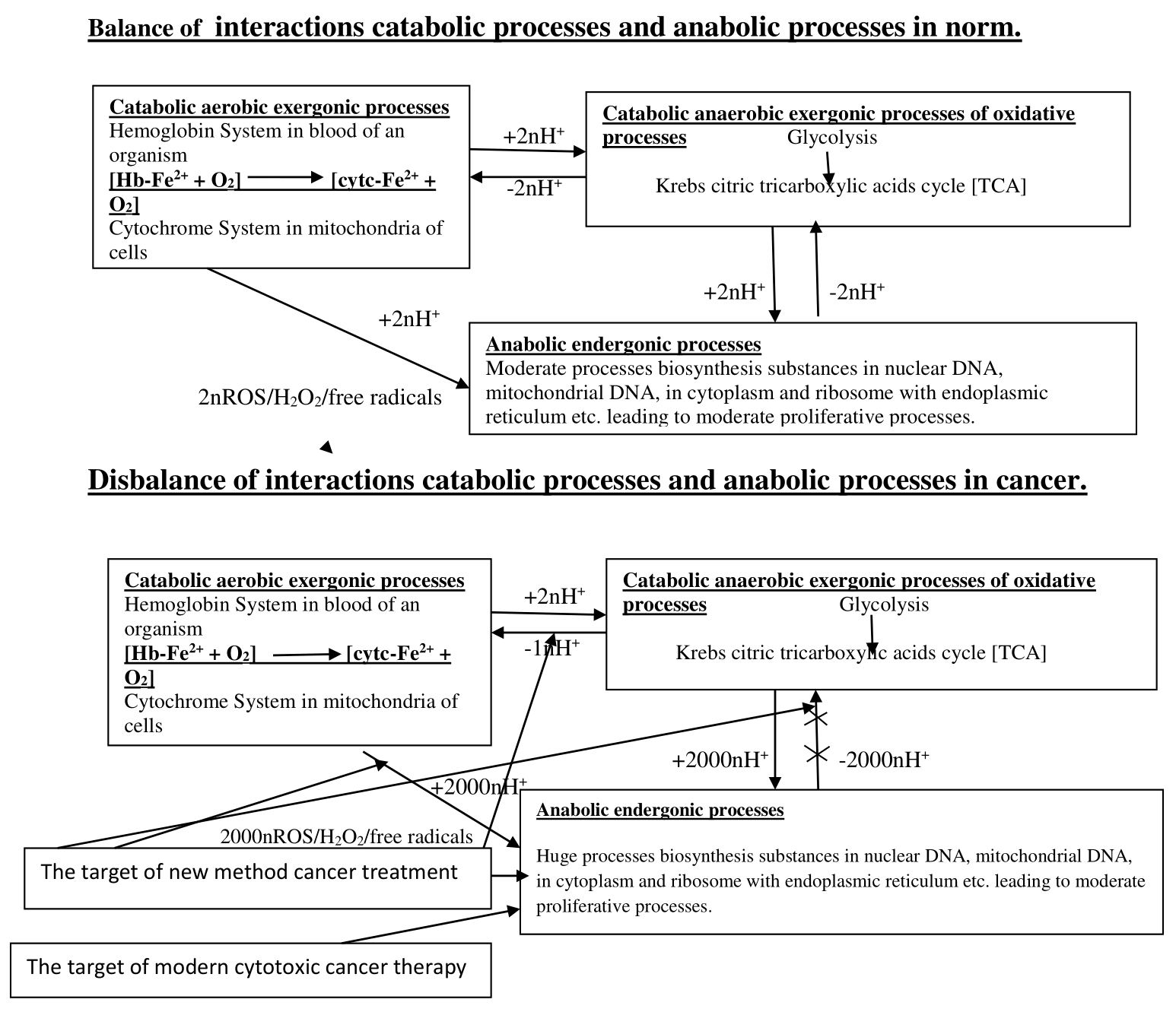
Symbols: Hb-Fe3+ , hemoglobin; cytc-Fe3+, cytochrome
Figure 5 The targets of both the new method cancer treatment and modern method of cancer treatment.
Hence expression Mitosis in normal cellular cycles of all cells stimulate T cells [T lymphocytes] via appearance produced immunoglobulins CTLA-4 and PD-1 exerting resonance waves of cellular capacitors T cells memory to learn and remember by reaction of resonance waves on orbital molecular functions of viral substances containing in separated haploid Meiosis phase according famous Schroedinger equation of molecular orbitals as a linear combination of atomic orbitals (LCAO-MO). Then T cells memory transits data of viral cells‘ substances to → T cells helper, and T cells helper transits data of viral cells‘ substances to → T cells killer and B cells for production antibodies against cancer viral cells that destruct substances of viral pluripotent stem cells. Just it exerts mechanisms operation together of immune T cells and macrophages with monocytes in autophagy for cleaning Internal Energy of a himan organism from infected with v-oncogenes of microRNAs in the dead cancer cells causing by autoimmune antibodies. Thus the mechanism of Cancer Immunotherapy prevent possible relapse cancer disease for successful termination of cancer therapy by new method of cancer treatment. Thus such basic phenomena of the new method of cancer treatment are showed by following inhibitions:1,78–80
Thus inhibition of Warburg effect by “Prolonged medical Starvation (during 42-45 days)” leads to cancer cells’ depressions which are determined by following changes:
The normalized balance anabolic processes & catabolic processes end cancerous overloaded of Nodal point in Acetyl-CoA of bifurcation anabolic processes and catabolic processes [NPBab]“ that ends mechanisms of metastases and non-healing tgumor ulcers.
Benefits of the use “Prolonged medical Starvation” in Cancer therapy: “Prolonged medical Starvation” contributes to depression of cancer tumor metabolism that helps for efficient anticancer therapy with considerably decreased dosage of cytotoxic substances or drugs. Such approach to anticancer chemotherapy prevents damage Internal Energy and Internal Medium both an organism and cells of an organism, preventing damage of immune and hormonal systems as the links of defensive mechanism in regulative system of an organism. Immune and hormonal systems as the links of system stability Internal Energy and Internal Medium an organism prevents both recurrence of cancer disease after long anticancer chemotherapy and resistance to anticancer drugs which occur due to intensive anticancer chemotherapy with large dosage of cytotoxic drugs due to intrusion into Internal Energy and Internal Medium of an organism. Thus therapeutic targets of new method cancer treatment using combination immunotherapy with small dosage weak cytotoxic substances are more efficient than modern methods cancer treatments with large dosage cytotoxic drugs (Figure 5).79
Immunotherapy of cancer diseases must not be combined with chemotherapy using large dosage cytotoxic drugs because the chemotherapy with large dosage cytotoxic drugs destruct mechanism of immunotherapy of cancer disease and is incompatibility with mechanisms of Cancer immunotherapy of the new method cancer treatment!!
It must begin treatment of cancer disease from Prolonged Starvation 42 – 45 days with very small quantity extraction of cytotoxic substance. Then after going out of Prolonged Starvation 42 – 45 days through one month, it will be able to use prophylactic treatment with less of middle dose of cytotoxic drug
This article is dedicated to the memory of my daughter T.M. Ponizovska.
The authors declare that there are no conflicts of interest.

©2024 Ponizovskiy. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.