International Journal of
eISSN: 2576-4454


Review Article Volume 4 Issue 5
1Professor, Deputy Director for Research and Development, KUD Industries P.N. Ltd - Israel Technology Research Center, Russia
2Director, KUD Industries P.N. Ltd - Israel Technology Research Center, Russia
Correspondence: Kudryavtsev P, Professor, Deputy Director for Research and Development, KUD Industries P.N. Ltd - Israel Technology Research Center, Russia
Received: August 20, 2020 | Published: September 11, 2020
Citation: Kudryavtsev P, Kudryavtsev N. Treatment of natural surface waters using new composite flocculants-coagulants. Int J Hydro. 2020;4(5):211-227. DOI: 10.15406/ijh.2020.04.00248
Coagulation is an essential process in the treatment of water and industrial wastewater. In the field of drinking water treatment since ancient times, water purification using coagulants using various substances has been practiced. Coagulation is the most common method of purification of natural and wastewaters from the bulk of colloidal, finely dispersed, and partially dissolved contaminants. The characteristics of the new composite flocculants-coagulants ASFC and ISFC developed by the authors are presented in comparison with conventional aluminum and iron salts. The titration method was used to study the behavior of coagulant flocculants at different pH. The titration method was used to study the behavior of coagulant flocculants at different pH. The ζ-potentials and isoelectric points for aluminum hydroxide obtained by hydrolysis of a coagulant are determined. The nature of the interaction of active silicic acid and the products of hydrolysis of aluminum salts as components of composite flocculants-coagulants is shown. The characteristics of natural waters are given, on which the efficiency of using the obtained flocculants-coagulants is investigated. A comparison of the coagulating ability of coagulants in water purification with high color from the Orsha River and with medium color from the Volga and Tvertsa rivers is presented.
Keywords: coagulation, flocculation, hydrolysis, compositional flocculants-coagulants, ASFC, ISFC, water purification
Coagulation is an essential process in the treatment of natural water and industrial wastewater. In the field of drinking water treatment since ancient times, water purification using coagulants using various substances has been practiced. Coagulation is the most common method of purification of natural and wastewaters from the bulk of colloidal, finely dispersed, and partially dissolved contaminants. Several factors determine the widespread use of mineral coagulants for water treatment:
The scale of application of the coagulation method has increased in recent years and, judging by the forecasts, will increase. Therefore, the search for ways to improve this method is relevant. Aluminum and iron salts are widely used as coagulants in the treatment of natural and wastewaters, as well as in several other industries. They are useful in removing a wide range of impurities from water, including colloidal particles, dissolved organic matter, petroleum products, and heavy metal salts. Their action is mainly due to two different mechanisms:
Many chemicals are used to produce potable water and in the treatment of wastewater effluents. In potable water treatment, chemicals such as inorganic salts and polymeric organic coagulants are used for primary coagulation, as coagulant aids, and for sludge dewatering; lime and soda ash allowed for pH correction and water stabilization.1 The relative contribution of these mechanisms depends on factors such as pH, coagulant dosage, solution ionic strength, type, and concentration of harmful impurities. Alternative coagulants are products based on the creation of pre-hydrolyzed forms of aluminum and iron. These products are, in many cases, more effective than traditional coagulants. The next promising direction in the production of new substances for reagent water purification is composite materials combining coagulation and flocculation functions. One of such directions in the intensification of coagulation water purification technology is the use of composite reagents, which include the studied coagulants ASFC and ISFC. Composite coagulants have an additive and synergistic effect, which will increase the efficiency of water treatment, reduce costs, and the number of reagents used, simplify the technology of their use.
Low molecular weight inorganic or organic electrolytes leading to particle aggregation are called coagulators. A case of coagulants is coagulants — hydrolyzable salts, for example, sulfates and halides of multiply charged cations (aluminum, iron, titanium, and others). Flocculants are inorganic or organic high molecular weight chemical compounds that facilitate the formation of aggregates during the deposition of particles of a dispersed system. These substances act by combining several particles with the help of polymer macromolecules, which are adsorbed or chemically bound to the surface of the particles of the dispersed system.1
Kruchinina N.E. and Kim V. et al. developed a method for treating nepheline with sulfuric acid,2,3 because of which a liquid aluminum-silicon flocculant-coagulant (ASFC) is formed. This method of producing aluminosilicate coagulant, in which the aluminosilicate raw material is treated with a solution of sulfuric acid and a stabilizing additive is introduced into the resulting solution. The disadvantages of this method are the insufficient shelf life of the finished product, the complexity of the process, the use of stabilizing polymer additives, and significant energy consumption in its manufacture.
Alumina-silicon flocculant-coagulant ASFC is one of the few binary compositions that include only inorganic components: coagulant - aluminum sulfate and anionic flocculant - active silicic acid. The action of ASFC is based on the interaction of its primary ingredients. The principal components are coagulant - a compound of aluminum and flocculant - active silicic acid. During the interaction of the starting components, complex compounds are formed with higher flocculating ability. The resulting products are zeolite-like nanoscale structures with a developed sorption surface. This combination of starting products leads to a synergistic effect, which consists in increasing the effectiveness of the impact because of the integration of individual processes into a single system. The water purification mechanism is implemented due to the volumetric sorption of pollutants on self-organizing aluminum-silicon complexes.
Due to the instability of the product obtained by dissolving nepheline raw materials, the authors were faced with the task of developing a method for producing aluminum-silicon flocculant-coagulant - in the form of a crystalline product, which has higher stability, long shelf life. It should be easy to manufacture and economical during transportation, have a higher content of the active component. It should also be useful and easy to use for wastewater treatment. The authors managed to solve this problem by combining in the solid phase all the active components of this material.4 The advanced synthesis method was based on the principles underlying the well-known matrix isolation method, which was developed at the end of the last century. The matrix isolation method allows you to freeze and study reactive particles with a short lifetime in an inert solid matrix. As is known, intermolecular interaction is most pronounced in the case of chemically active particles. Such particles are most atoms, free radicals, and molecules that are in the monomeric state only at high temperatures. Such particles can only be investigated in the gas phase and only at low concentrations. However, even under such extreme conditions, some particles are so reactive that they can only exist for a short time after formation. Therefore, the study of their molecular properties is complicated.
The matrix isolation method arose as an attempt to overcome the above difficulties in the study of reactive molecules. It consists of freezing the studied molecules in a harsh environment (matrix) of a chemically inert substance at low temperatures. The rigidity of the matrix prevents the diffusion of active molecules, i.e., complicates their interaction with other similar particles. In turn, the inertness of the matrix substance is necessary to avoid the interaction of active particles with the matrix.5 This situation is also observed upon receipt of aluminum-silicon flocculants-coagulants. The use of such technological methods made it possible to "freeze" and isolate the components of the flocculant-coagulant in the solid phase matrix — acid salts of aluminum sulfate and active silicic acid, which is nanodispersed or in the form of polymeric alkali metal silicates. A quick translation of the active components into a solid-state makes it possible to drastically reduce the rates of diffusion processes and, at the same time, maintain the activity of the material. In the development of this method, not only aluminum-silicon flocculants-coagulants but also silicon-iron materials were obtained. Such materials were given the common name – ISFC.9 A whole series of works,6–20 was devoted to studying the influence of the conditions of the synthesis of these materials and their use for treating wastewater from oil products and salts of heavy metals.
In this regard, this work aims to compare the effectiveness of the use of composite reagents ASFC and ISFC and traditional coagulants - aluminum sulfate and vitriol, which are components of the studied composite coagulants, for the purification of natural waters.
Hydrolyzing metal salts based on aluminum or iron are very widely used as coagulants in water treatment. The ancient Romans were also familiar with alum. Alum or aluminum sulfate has been used by them for water purification since ancient times and was first mentioned by Pliny (about 77 A.D.).21 By 1757, alum was used as a coagulant in water treatment in England, and in 1881 more formally in water treatment for public purposes.22 In modern water treatment, coagulation and flocculation are still essential steps in the treatment of various types of natural and wastewater. The trivalent aluminum and iron salts began to be used to coagulate dispersed contaminants in almost all water treatment systems after it was found that the coagulation effect increases with increasing valency of the coagulating ion. To date, much experimental material has been accumulated on the use of coagulants for the purification of natural waters, which has been reflected in numerous publications and monographs.23–34 The classic coagulant for the purification of natural waters, which until recently was used on water pipelines in Russia, is aluminum sulfate. The use of aluminum sulfate is due to its high coagulating, adsorption, and precipitation ability of this reagent and its hydrolysis products concerning most pollution of natural waters, availability, and low cost. However, aluminum sulfate also has several disadvantages, the main of which is low efficiency and high residual aluminum content at low temperatures. Iron (II) sulfate is used less frequently in water treatment due to the need to create special conditions (introducing an oxidizing agent, alkalization) to convert ferrous iron to ferric and maintain a normalized content of residual iron in purified water.35
When using aluminum sulfate (AS), to increase the efficiency of water purification, various flocculants are used. The most widely used flocculants are active silicic acid and polyacrylamide (PAA).36 In recent years, with an increase in the market assortment of flocculants, cationic organic coagulants, for example, VPK 402, and high molecular weight cationic flocculants, for instance, Praestol 650, have been used in water treatment.37 Active silicic acid (ASC) has several advantages over PAA, the main of which is the absence of toxicity and secondary pollution of water with organic substances, the availability of an accessible raw material base. Therefore, in water treatment, starting from the 60-s, aluminum sulfate began to be used in combination with active silicic acid.36 The main disadvantage of active silicic acid, the solutions of which exhibit the properties of an anionic polyelectrolyte, is instability and a tendency to gel formation during storage.36 Therefore, active silicic acid in the form of dilute aqueous solutions is obtained directly at the treatment plant by the interaction (activation) of sodium silicate solutions with acids or acid salts of aluminum or iron. The process takes place in two stages. First, an activation reaction of liquid glass having an alkaline reaction with acidic reagents to produce silicic acid occurs. Then, the resulting silicic acid H4SiO4, due to the presence of reactive silanol groups-SiOH, enters the polycondensation reaction according to the scheme:
(1)
As a result, polysilicic acids are formed with a linear, branched, or mixed structure, which some authors consider as colloidal particles, prone to coagulation during storage, and others as soluble polyelectrolytes, capable of forming insoluble network structures and gel formation. Therefore, the polycondensation process is often called the process of maturation of the active silicic acid sol. In most recommendations for producing active silicic acid, a 1.2÷1.7% sodium silicate solution (according to SiO2) is used, and it is neutralized with 20% sulfuric acid or 1.5÷2.5% aluminum sulfate to pH 7÷8. The process of polycondensation - maturation of sols, which is controlled by increasing the viscosity or increasing the turbidity of the solution, ends in 60-120 minutes. After completion of the process, to extend the shelf life of activated silicic acid, the sol is diluted with water to obtain a solution with a concentration of 0.5÷0.75% for SiO2.The polycondensation rate depends on pH and is minimal at pH 2 ÷ 3. Coagulation resistance of active silicic acid solution is maximum at pH> 7.5. The gelation rate is highest at a pH value of 5.5 ÷ 6.0. By choosing the appropriate conditions and using special additives (stabilizers), you can increase the stability and concentration of solutions of active silicic acid.
More promising is the use of a mixture of reagents consisting of aluminum sulfate and sodium silicate. It is recommended that a 1% solution of sodium silicate be mixed with a 1% solution of aluminum sulfate in a ratio of 1:4, and the resulting solution immediately used as a coagulant. In recent years, powder and liquid silicon and aluminum-silicon coagulants with enhanced stability and concentration have been developed. For example, the "SIZOL" series inorganic reagents based on stabilized silica sol (20 % SiO2) were developed at the Institute of Bioorganic Chemistry and Petrochemistry of the Ukrainian Academy of Sciences. The prepared reagent is stable in pH regions close to neutral, when, as an unstabilized silicic acid sol, it quickly turns into a gel.38
As already noted above, the authors of2,3,39 developed a liquid composite aluminum-silicon flocculant-coagulant (ASFC). It was obtained from the alumina-silicate mineral nepheline by treating it with a solution of sulfuric acid. The product thus purchased can be considered as a mixture of aluminum sulfate and active silicic acid, or as aluminum poly-hydroxy silicate sulfate. It is formed because of the polymerization of primary aluminum hydroxo complexes. Such polynuclear hydroxocomplexes40 are formed in solutions of acidic and basic aluminum salts, due to the high complexing properties of aluminum. To date, hydroxocomplexes with an aluminum atom content of 6 to 54 have been identified: , , , . In polynuclear hydroxocomplexes, aluminum ions are interconnected by bridging O.H. groups — ol-bonds or O-bridges — oxo bonds. However, not all such compounds are formed during the hydrolysis of aluminum salts.41,42 A diagram of the regions of existence for various states of aluminum (III) ion, related to the ionic strength of 1.0 mol/kg chloride, is shown in Figure 1, where the supposed solid phase is crystalline gibbsite, Al(OH)3(s). This diagram shows that low ionic strength, as well as high temperature, contribute to the formation of monomer particles of metal ions. The picture also shows that the predominant solid phase, primarily due to unsaturation, is amorphous aluminum hydroxide Al(OH)3(am). It was found that, unlike many other metal ions, for aluminum, there is a limited region where the polymer products of aluminum (III) hydrolysis predominate.

Figure 1 A diagram of the regions of existence for various states of an aluminum (III) ion at 25 ℃, in a solution with an ionic strength of 1.0 mol NaCl /kg.
The data presented in43 show that the solubility constant for Al(OH)3(am)pKs=10.38 in a 0.1M sodium chloride solution. The solubility for crystalline gibbsite is approximately two orders of magnitude lower (pKs=8.34). The assumption that Al(OH)3(am) is a solid phase leads to a relatively large stability region for polymer ions [Al13(OH)32]7+. It was shown in44 that the dominant components of solutions of aluminum (III) salts were ionsAl3+, [AlOH]2+, [Al13(OH)32]7+ and [Al(H2O)2(OH)4]-. This behavior is consistent with the fact that the other two polymer species are only secondary types of ions.45 A more detailed review of studies on the state of aluminum (III) ions in aqueous solutions during hydrolysis is presented in our work.46 The stability and solubility constants obtained at 25°C for zero ionic strength were used to create a diagram of the regions of existence for various states of the iron (II) ion. The state diagram of the iron (II) ion is shown in Figure 2. The picture assumes that the solid phase dominates. However, this solid-phase controls solubility only in a relatively small region.43 The hydrolysis of iron salts in solutions was studied by cation exchange, and the hydrolysis constants pK1=6.90 and pK2=8.25 were determined. Based on these data, a distribution diagram of the iron (II) hydroxocomplexes is plotted versus the pH of the solution (Figure 2). The hydrolysis of iron (II) in the first stage begins at pH≈5 and proceeds most fully; The pH of the onset of precipitation of iron (II) hydroxide is pH≈7.0, and the pH of complete precipitation is 9–14. In dilute solutions of iron (II) salts, hydrolysis proceeds according to the following schemes:
(2)
(3)
(4)
Active silicic acid is the most common inorganic flocculant. It is obtained by condensation of low molecular weight silicic acids or their sparingly soluble salts. Active silicic acid is an anionic polyelectrolyte. Such parameters as the macromolecule's degree of polymerization, particle size, isoelectric point characterize the properties of active silicic acid solutions. They depend on the methods for preparing these solutions, the duration and storage conditions, and other factors. The preparation of active silicic acid consists of three stages.47
(5)
(6)
(7)
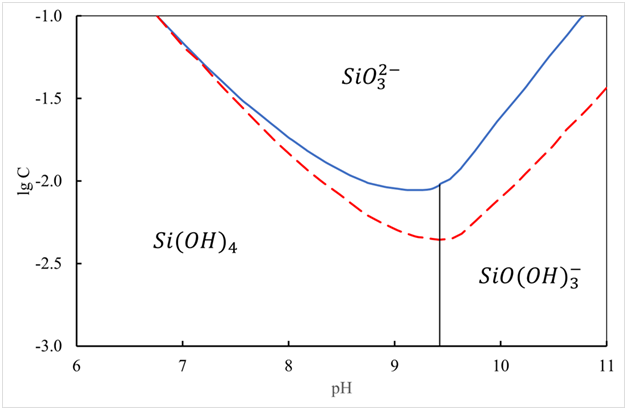
Figure 3 Diagram of regions of existence for various states of a silicon (IV) ion at 25 ℃. The dashed curve corresponds to the data for quartz (pKs = 4). Solid for amorphous silica (pKs = 2.76).
At pH 10.5, the total concentration of soluble silica can be explained by the presence of and - and, probably, a small amount of ions. Thus, no other ionic species can be present in appreciable amounts in such solutions.
The polymerization of active silicic acid begins in the region of pH 2-7. Its polymerization in solution in the areas of pH below and above 7 must be considered separately for the following reasons49
Since composite flocculants-coagulants of the ASFC type consist of two main components of active silicic acid and aluminum salt, it is necessary to consider the issues of their interaction with aqueous solutions. In his classic monograph,50 Euler noted that monomeric silica reacts with A13+ ions and most effectively precipitates at pH 9. If the reaction of monomeric silica Si (OH)4 with A13+ ions takes a long time at 25 °C, then aluminum silicate is formed in the colloidal form with a composition corresponding to the mineral galloisite:
(8)
Monomeric silica is strongly adsorbed on the surface of aluminum hydroxide. As a result of the reaction between and several layers of silica are formed on the surface of aluminum hydroxide. In this case, a decrease in the pH of the suspension is observed. The formation of the first layer is fast, but the second and third layers are completed much more slowly. It is likely that the diffusion of A13+ or ions should occur from the surface of the solid phase - with the formation of silica-rich alumina. The relatively small content of aluminum ions in the silica layer nevertheless markedly reduces the solubility of such a layer. The relatively low content of aluminum ions in the silica layer. however, significantly reduces the solubility of such a layer. This effect can explain the deposition of SiO2 from an unsaturated solution. The main reactive species are ions. The reaction with the monomer requires the presence of A13+ ions or polybasic aluminum ions.51
Given the above processes, it must be emphasized that the disadvantage of liquid ASFC is the ability to irreversible gelation during storage and loss of coagulating properties. More promising is the use of silicon-containing coagulants in the form of a powder mixture of sodium silicate and aluminum sulfate, which significantly simplifies the application technology and allows you to increase the shelf life of working solutions.41 Such powder reagents include coagulants ASFC and ISFC developed by the authors of,4,9 the coagulating properties of which were studied during this work.
In the present work, well-known standard methods of research and analysis were used. The reliable and reproducible results were obtainedUsing methods of potentiometry, turbidimetry, pH-metry, spectrophotometry.
The quantitative determination of the effectiveness of the use of inorganic coagulants for the purification of natural waters was carried out according to general indicators of water quality. All analyses were carried out following standard methods. The hydrogen index (pH) was measured using the HI-5222 ionomer and by the procedure described in.44 The HI-5222 ionomer is a professional bench-top device with a color graphical LC-display for pH measurement (with calibration test), redox potential, with the possibility of using ion-selective electrodes and temperature measurement. For calibration, commercially available certified reference samples of reference buffer solutions were used. Titration of flocculant coagulants was carried out using the indicated ionomer, combined with Automatic potentiometric titrator - HI-931.
The optical density determined the turbidity and color of natural water on a KFK-2 photo colorimeter at X = 540 nm and 400nm, respectively, in a 50mm cuvette according to the procedure described in.52 Chromaticity was determined in samples of purified water after filtering through a blue-ribbon paper filter. The content of residual aluminum was determined by the spectrophotometric method according to the standard method described in.52 The process is based on the ability of aluminum to form an orange-red lacquer with Aluminone, which is a complex compound. The reaction is carried out in a slightly acidic medium at pH 4.5 in the presence of ammonium sulfate as a stabilizer for the color of the varnish. The resulting solution measures the absorption of light at a wavelength of 525 ÷ 540nm. The detection limit of aluminum is 0.02mg/L, with a sample volume of 25cm3. The range of measured concentrations is 0.04 ÷ 0.56mg/L.
The iron content was determined according to.53 The method is based on the formation in a weakly acidic medium of a red-colored complex compound of ferric ions with sulfosalicylic acid. The resulting solution measures the absorption of light at a wavelength of 490÷500nm. In an alkaline environment (pH=8÷11.5), sulfosalicylic acid reacts with ferric and ferrous ions to form a yellow complex, the concentration of which is determined by the absorption of light at a wavelength of 400÷420nm. The content of ferrous iron is found by difference. The sensitivity of the method is 0.05mg/l Fe. Since coagulation water treatment at water treatment plants is usually carried out according to a two-stage scheme: coagulation with sedimentation and post-treatment by filtration, coagulation was carried out by trial coagulation. The method of trial coagulation allows you to choose a coagulant or flocculant, determine its optimal dose and effectiveness for the treatment of the studied water. The essence of the trial coagulation method is to treat the wastewater with reagents in cylinders or glasses under a specific standard mixing and flocculation mode or under conditions simulating coagulation treatment at existing treatment facilities.
Mixing is usually carried out with a mechanical stirrer, first quickly to evenly distribute the reagent in the water, then slowly to create large, rapidly settling flakes of coagulated contaminants. For this purpose, a particular test coagulation unit is used: "Drop," which allows us to simultaneously process six samples of wastewater and mix with mechanical stirrers with adjustable speed in the range from 10 to 200rpm.37,42
Mixing was carried out for one minute at an average velocity gradient of G=500s-1, flocculation for 5minutes at G=50s-1. Settling was carried out for 5 min to isolate contaminants with a hydraulic fineness of more than 0.03mm/s. It is known42 that the intensity of mixing of reagents with water is characterized by a velocity gradient G, which are determined from the following expression:
(9)
Where E is the energy spent on mixing, J; η is the dynamic viscosity of water, Pa s; T is the duration of mixing, s; V is the volume of water in the mixer, m3. For laboratory coagulation water purification, 0.1% solutions of coagulants in distilled water were used. The experiments were carried out on real river water with different initial color.
Characteristics of the coagulants studied
We used the coagulant ASFC, which is an aluminum-silicon flocculant coagulant of the formula 4.4Na2O∙Al2O3∙5.1SiO2∙6.6SO3∙4H2O with a molecular weight of 1281.6 (T.U. 26351-0013578-61-08) and iron-silicon flocculant coagulant ISFC The iron-based ferrous sulfate of formula 10Na2O∙FeO∙5.2SiO2∙2SO3∙8H2O with a molecular weight of 1308.31. The characteristics of the tested coagulants are presented in Table 1. A coagulant working solution was prepared by dissolving its sample, weighed on an analytical balance, in distilled water to a concentration of 1%. The active substance calculated the dosage of the reagent. In calculations of the concentration and dosage of coagulant and flocculant, the humidity was not considered since the reagents were stored in a dry place in hermetically sealed containers. When preparing working solutions of coagulants, it was found that coagulants are not completely dissolved in water. The insoluble part quickly settles, and the coagulant solutions become transparent in appearance and do not change during storage.54
|
Coagulant |
The content of the basic substance, oxide, % |
SiliconOxideContent, % |
pH of 0.1% solution (by Al or Fe oxide) |
|
ASFC |
6.04 |
18.2 |
1.97 |
|
Aluminum sulphate |
15.3 |
- |
2.7 |
|
ISFC |
3.72 |
16.1 |
2.26 |
|
Ironsulfate |
25.8 |
- |
2.77 |
Table 1 Characteristics of the investigated reagents
Titration of coagulant flocculants
Potentiometric titration curves, which are presented in Figures 4&5, were taken to characterize the properties of aqueous solutions of coagulants. As follows from Figure 4, on the potentiometric titration curves of composite coagulants, as well as the initial sulfate salts of aluminum and iron, there is a characteristic kink corresponding to the formation of aluminum or iron hydroxides. The equivalent point of the creation of aluminum hydroxide is 6.8÷7.0 for aluminum-containing coagulants.The formation of iron hydroxide occurs in the pH range of 9.6-9.8 for iron-containing coagulants. The second characteristic kink, which is especially clearly visible on the potentiometric titration curve of iron-containing coagulants with an acid equivalence point at pH=4.5, obviously corresponds to the formation of sodium silicate from silicic acid. Also, it is impossible to exclude the possibility of forming aluminum or iron silicates at this point, since under these conditions, their simple cationic forms are still present following the data shown in Figures 4&5.
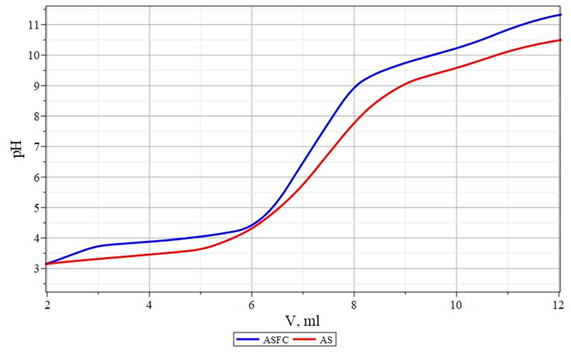
Figure 4 Potentiometric titration curves of 0.1% coagulant solutions with 0.1 N NaOH solution. ASFC is a flocculant coagulant of ASFC. AS - aluminum sulfate.
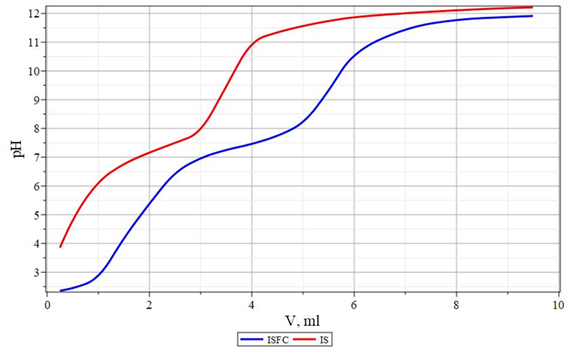
Figure 5 Potentiometric titration curves of 0.1% coagulant solutions with 0.1 N NaOH solution. ISFC - iron-silicon flocculant coagulant. AS - iron (II) sulfate.
Titration of an aluminum salt solution with a base usually gives a curve like a curve in Figure 4. On this curve, four areas can be distinguished depending on the amount of support added. In the first region, at the beginning of the curve, the base neutralizes the free acid formed during spontaneous hydrolysis, which leads to a rapid increase in pH. Above approximately pH 4, hydrolyzed particles are formed, and there is an expanded portion of the curve where the pH increases slowly since the added base is consumed to hydrolyze the aluminum salt. In this area, large amounts of polymer particles and hydroxocomplexes can form, which become the predominant substances in the solution with a large quantity of alkali added. In a titration, the addition of a base can lead to various effects, depending on the mixing conditions. For example, local supersaturation and precipitation of amorphous hydroxide or excessive formation of the - ion. These conditions may also contribute to the formation of the [Al13(OH)32]7+, polymer, although there is no clear unambiguity.
A further increase in the amount of added alkali gives a third region in which a small shoulder appears on the curve after a sharp rise in pH. In this region, a solution is oversaturated concerning amorphous Al(OH)3 and rapidly precipitates. This arm is enhanced in chloride solutions and weakens in the presence of highly charged anions, such as sulfate, which can facilitate the precipitation of hydroxide.55 In the case of ASFC, the reduction and shift of this arm is facilitated not only by the presence of sulfate ions , but also by the presence of silicate ions , which are included in its composition.
In the fourth region, the added base reduces the positive surface charge of the colloidal hydroxide particles and visible precipitates form. Further addition of the base gives a rapid increase in pH for the light of the dissolution of aluminum hydroxide and the formation of aluminate ions and . A similar situation is also observed for ISFC.The difference between ISFC and ASFC is the shift of the inflection on the titration curves to the acidic region. However, the third region on this curve appears more clearly than in the case of ASFC. Their behavior is because of sulfate ions and silicate ions , which are its constituents, have a smaller effect on the formed iron (II) hydroxide . The resulting iron (II) hydroxide is relatively well soluble in water.Therefore, it is in a dissociated state, and, as a result, flocculation does not occur in water. Therefore, for the complete coagulation when using iron sulfate, the resulting iron (II) hydroxide must be converted to iron (III) hydroxide. The latter can occur during the treatment of purified oxygenated water:
(10)
It was found that flakes of iron hydroxide are most efficiently formed at pH 5-7, and best of all at pH 6.1-6.5. In addition to this process, an additional oxidation of iron (II) hydroxide with oxygen dissolved in water with the formation of iron (III) hydroxide occurs in an alkaline environment.
Fe(OH)_2+H_2 O→Fe(OH)_3+H^++e^-
φ=0.271-0.0591∙pH (11)
This process proceeds most easily in the alkaline region and contributes to the additional consumption of alkali. In the fourth region, the addition of a base also reduces the positive surface charge of the particles of colloidal iron hydroxide, and sediments are formed that actively interact with the flocculant colloidal silicon oxide.
Changing the buffer capacity of coagulant solutions
During potentiometric titration of coagulant solutions, it was found that they behave as buffer solutions, that is, solutions with a stable concentration of hydrogen ions. Studying the dependence of the buffer capacity of coagulant solutions on their pH allows us to obtain more detailed information about the structure of substances formed in coagulation processes. In coagulant solutions, as in buffer solutions, the pH changes little when small amounts of strong base or strong acid are added to them. The prepared solutions retain a certain pH only up to a certain amount of added acid or base, which is associated with a change in the concentration of its components. Its buffer capacity determines the ability of a buffer solution to maintain its pH, that is, the amount of strong acid or alkali that must be added to a particular volume of the buffer solution so that its pH remains constant or changes within an acceptable value. The buffer capacity is higher, the higher the concentration of its components. Buffer capacity is a quantitative measure of resistance to changes in pH of a solution containing a buffering agent concerning changes in acid or alkali concentration. It can be defined as follows47,48
(12)
Where:the infinitely small amount of added alkali solution. Considering the charge symmetry concerning cations and anions, for acid titration, we have a similar equation:
(13)
Where - the infinitely small amount of acid solution added. represents an infinitesimal change in pH corresponding to a given amount of titrant solution added. Since the action of a base hydrolyzes coagulants, they can be considered as weak acids. With any determination, the buffer capacity for a weak acid H.A. with a dissociation constant can be expressed as49,50 as:
(14)
Where: - the activity of hydrogen ions, and - the total concentration of added acid. is the equilibrium constant of water ionization (ionic product of water), equal to . It should be noted that in solution H + ions exist in the form of hydroxonium ions .Further hydration of the hydroxonium ion has little effect on the equilibrium of dissociation, except for an extremely high concentration of acid.
This equation shows that there are three areas of increased buffer capacity. In the central region of the curve (5), the second term is dominant; therefore, expression (5) takes the form:
(15)
The buffer capacity increases to a local maximum at . For coagulants, the height of this peak depends on factors such as concentration, hydrolysis mechanism, and values. The buffer capacity is negligible when the concentration of [H.A.] buffering agent is minimal and increases with increasing concentration of the buffering agent.48 Some authors show only this area on the graphs of buffer capacity.47
The buffer capacity drops when deviating from the maximum value at .
For strongly acidic solutions in the left part of the curve, the first term in equation (14) dominates, and the buffer capacity increases exponentially with decreasing pH:
(16)
This situation is explained by the fact that the second and third terms become insignificant at shallow pH values. This condition is independent of the presence or absence of a buffering agent and is a natural property of the pH. In the case of strongly alkaline solutions, the right side of the curve prevails. Under these conditions, it is determined by the third term and dominates equation (14), and the buffer capacity under these conditions increases exponentially with increasing pH:
β≈〖10〗^(pH-K_w ) (17)
Equation (17) is a consequence of the fact that the first and second terms become insignificant at an extremely highpH. This condition is also independent of the presence or absence of a buffering agent. Curves of changes in the buffer capacity of coagulant solutions are shown in Figures 6&7. The parameters of the buffer capacity calculated on their basis are presented in Table 2. During titration of coagulant solutions, not only pH measurement and determination of buffer capacity were carried out, but also the change in turbidity of the solution was measured by turbidimetry to control the process of solid phase formation.
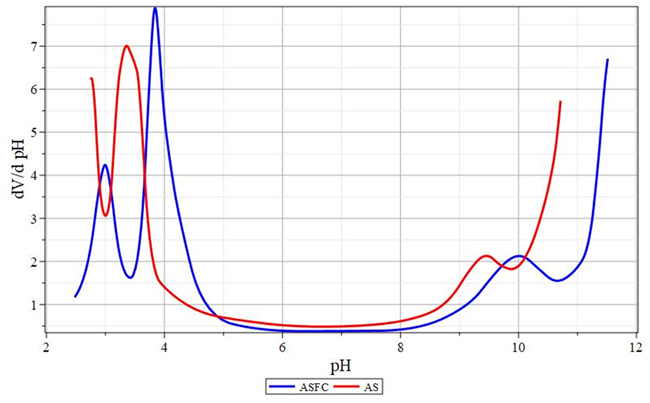
Figure 6 Curves of changes in the buffer capacity of coagulant solutions.
ASFC is a flocculant. AS - aluminum sulfate.
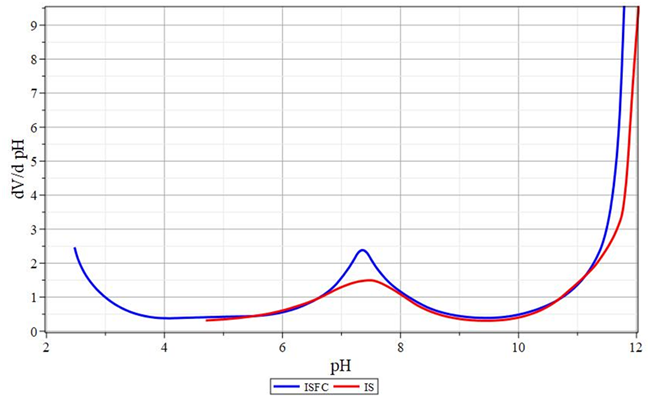
Figure 7 Curves of changes in the buffer capacity of coagulant solutions.
ISFC - flocculant. IS - iron (II) sulfate.
|
Coagulant |
pH value corresponding to the maximum |
|||||||
|
1 |
2 |
3 |
4 |
|||||
|
pHmax |
β |
pHmax |
β |
pHmax |
β |
pHmax |
β |
|
|
ASFC |
2.97 |
4.22 |
3.83 |
7.77 |
9.99 |
2.11 |
>11.5 |
>6.7 |
|
Aluminum sulphate |
2.73 |
6.3 |
3.35 |
7.00 |
9.44 |
2.14 |
>10.7 |
>5.8 |
|
ISFC |
<2.5 |
>2.5 |
- |
- |
7.34 |
2.41 |
>11.8 |
>9.5 |
|
Ironsulfate |
|
|
- |
- |
7.47 |
1.49 |
>12 |
>9.5 |
Table 2 The position of the maxima on the curves of changes in the buffer capacity of coagulant solutions
Turbidimetric studies of flocculant coagulants
To characterize the properties of aqueous solutions of coagulants, the curves of turbidimetric titration, which are presented in Figure 8, were taken. The turbidimetric analysis uses the phenomenon of light scattering by solid particles in suspension in solution.51 A stream of light illuminates the sample with intensity , and then, as in molecular absorption spectroscopy, the intensity of transmitted radiation is measured, or the intensity of radiation scattered at a certain angle is determined (for example, at 90 °). With an increase in the number of suspension particles, the ratio decreases, and relations of the form increase, in any case, to moderate concentrations. For very dilute suspensions, the measurement at an angle is much more sensitive than the measurements when the radiation source and receiver are on the same line since, in this case, you can observe weak scattered light against a dark background.
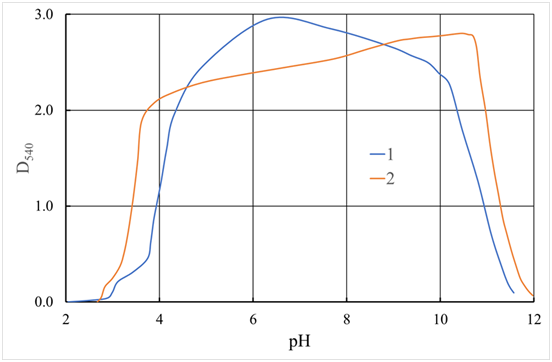
Figure 8 Curves of turbidimetric titration of 0.1% coagulant solutions of 0.1 N NaOH.
1 - ASFC; 2 - Aluminum sulfate
D540 - the optical density of the solution when using radiation with a wavelength of λ = 540 nm.
The intensity of the incident light flux is attenuated because of its dispersion by the dispersed system. If scattered light is taken to be fictitiously absorbed, then a simple relation can be obtained that is like the Lambert-Bouguer-Beer law for the absorption of light by molecular solutions. The attenuation of the light intensity dI is proportional to the intensity of the incident light passing through the layer of the system under study with a thickness dx
dI/dx=τI (18)
Where: τ is the proportionality coefficient characterizing the ability of the system to scatter light, it is called turbidity. For turbidimetric measurements, you can use any photometer or spectrophotometer. If the solvent and scattering particles are colorless, maximum sensitivity is achieved by using blue or near-ultraviolet radiation. For painted systems, the optimal wavelength must be selected experimentally. As a result of the integration of equation (18) in the range from to and, accordingly, from to , the thickness of the system layer, we obtain:
(19)
Where: τ - a constant depending on the geometry and properties of the system under study.
From equation (19), it follows that the turbidity () is a logarithmic dependence of the ratio of the intensities of the scattered and incident light, referred to the unit length of the ray of light passing through the sample.This ratio following the law of Rayleigh is equal to:
(20)
Where: d- the particle diameter; ν- the calculated concentration of dispersed particles in the solution; α- a constant depending on the nature of the dispersed system; c- a constant including constant parameters in the Rayleigh equation; λ- the wavelength of light incident on the cell; θ- the angle between the direction of light scattering and the direction of the incident light flux.
The Rayleigh formula for the intensity of light scattered by a unit volume of a dispersed system with spherical particles is valid for particles much smaller than the wavelength of the incident light. It is generally believed that the particle size should be no more than . Therefore, in experiments, radiation with a wavelength of nm is usually used. Curves of turbidimetric titration of solutions of ASFC and aluminum sulfate, alkali solution are presented in Figure 8. They show that for both coagulants, the hydrolysis products have approximately the same maximum optical density D540, i.e., the component composition of the ASFC does not significantly affect the optical density of the final products of the coagulant hydrolysis.
However, there are individual differences in their behavior. So, for aluminum sulfate, the maximum on this curve is reached at pH≈10.5. Also, the optical density increases almost linearly from pH = 4, although against the general background, these changes are insignificant. They can be associated with an increase in the particle size of aluminum hydroxide and a simultaneous decrease in their partial concentration per unit volume of the test solution. In this regard, the curves of turbidimetric titration of an ACPA solution have a different form. Two similar sections characterize them: the left in the range and the right . It can be assumed that these deviations and similar behavior are due to the presence of the second component of ASFC - active silicic acid. Judging by the appearance of the obtained dependencies, we can assume that a more detailed description of these dependencies can be obtained by constructing their derivatives concerningpH. The function graph for ASFC and aluminum sulfate is shown in Figure 9.56-60

Figure 9 Derivatives (dD_540)/dpH from the turbidimetric titration curves of 0.1% coagulant solutions.
Titrant 0.1 N NaOH. 1 - ASFC; 2 - Aluminum sulfate
D540 - the optical density of the solution when using radiation with a wavelength of λ = 540 nm.
The dependences shown in Figure 9 are remarkably similar to the pH dependences of the buffer capacity, although the extrema do not coincide with them. The position of the extrema in these dependencies is presented in Table 3. Comparing these data with the data in Table 2, we can see that in this case, the position of the extrema is shifted towards neutral pH values. This effect indicates the absence of a direct connection between the turbidity of the solutions and the hydrolytic reactions occurring in them.Also, it should be noted that extrema in the presented dependences are more extended for ASFC, compared with aluminum sulfate. It can be assumed that this effect is also associated with the impact of active silicic acid and is explained by the heterocoagulation nature of the deposition of aluminum hydroxide in its presence. However, there is also a significant difference between the characters of sedimentation turbidimetric curves. Figure 8 shows that the pH range for the deposition of aluminum sulfate also turned out to be slightly wider than this range for ASFC. On each side, it is about 0.5 pH units wider.
|
1 |
2 |
3 |
||||
|
pH |
pH |
pH |
||||
|
ASFC |
3.05 |
0.95 |
3.91 |
3.20 |
10.61 |
-1.88 |
|
Aluminum sulphate |
2.79 |
1.41 |
3.57 |
4.88 |
11.04 |
-3.49 |
Table 3 The position of the special points on the curves of turbidimetric titration, for 0.1% solutions of coagulants. Titrant 0.1 N NaOH
Determination of the ζ-potential and isoelectric point for aluminum hydroxide obtained by hydrolysis of a coagulant
As noted above, the coagulation process is highly dependent on the pH of the medium, and, first, it determines the charge of the particles of the suspension formed during coagulation. In this case, the H3O+ and O.H.– ions play the role of potential-determining ions on the hydroxide / aqueous solution interface. For example, at pH <pHiep, the appearance of a positive charge on aluminum hydroxide particles is due to the adsorption of hydrogen ions since, in this medium, they are potential-determining ions.61,62 Figure 9 shows the results of measuring the electrokinetic ζ-potential for a double electric layer and the average measured particle size of aluminum hydroxide during its formation from an aluminum salt depending on the pH of the medium. These data were obtained on a The NanoPlus instrument from Micromeritics Instrument Corp.60 The data presented in Figure 10 show that at pH near the isoelectric point, primary particles of aluminum hydroxide stick together, which is reflected in an increase in the measured particle sizes in the resulting suspension. In this case, the excessive adsorption of one of these ions determines the density and sign of the electric charge on the surface of the particles of the solid phase, which causes two effects. The first effect provides an increase in electrostatic repulsion, and the second leads to a decrease in interfacial tension. Both results contribute to the reduction in Gibbs energy, which leads to the occurrence of peptization processes and the destruction of the formed coagulate precipitate.
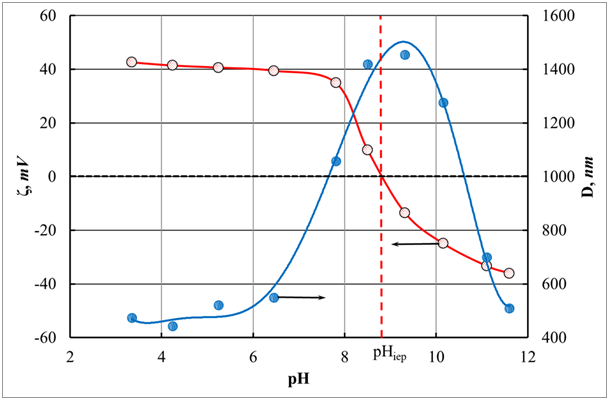
Figure 10 Change in the magnitude of the electrokinetic ζ-potential for a double electric layer and the average measured particle size of aluminum hydroxide as a function of pH.
pHiep is the isoelectric current (pHiep = 8.87).60
Interaction of active silicic acid and hydrolysis products of aluminum salts as components of composite flocculants-coagulants
The problem of joint coagulation of colloidal dispersions, consisting of two different types of colloidal particles, is of great practical importance and is of natural interest to many researchers.63,64 In this section, we consider the influence of the state of the individual components of composite flocculants-coagulants on the rate and nature of the interaction of active silicic acid and the products of hydrolysis of aluminum salts. As is known, the stability of colloids depends on the nature of the dispersed phase, charge and size of colloidal particles, pH and ionic strength of the solution, temperature, and other factors.51 Also, when two different dispersed phases interact in a solution, the ratio of sizes and masses of dispersed particles is of great importance.63 One of the important parameters determining the stability of sols is the charge of colloidal particles. The hydroxonium ions H3O+ and hydroxyl ions O.H.- are the potential determinants for colloidal dispersions of silicon and aluminum oxides, as well as for other dispersions of metal oxides and oxyhydrates.It is known that silica sol at pH=8.0 has a ζ potential equal to 25 mV, pH=2.2 corresponds to the point of zero charges, and at pH=1.0, the particles acquire a small positive charge. Alumozole at pH = 3.6 has ζ = +60 mV. At certain concentrations and particle sizes, the colloidal alumina solution is overly sensitive to changes in pH.
Moreover, the colloidal solution of silicon oxide is quite stable over a wide pH range. Therefore, the effect of the particle charge of a colloidal solution of silicon oxide, that is, its pH, on the stability of a system containing a mixture of the corresponding colloidal solutions was studied. Moreover, the parameters of the products of hydrolysis of aluminum salts were maintained at a constant level. The dependence of the lifetime of a mixed aluminosilicate disperse system on the charge (pH) of silica sol, and their ratio is shown in Figure 11. The maximum difference in charges causes rapid coagulation in a wide range of compositions of the mixed system (curve 3). The stability of the combined system increases with decreasing the pH of the silicate component (curves 1 and 2). This effect is explained by a decrease in the difference in charges of active silicic acid and the products of hydrolysis of aluminum salts.
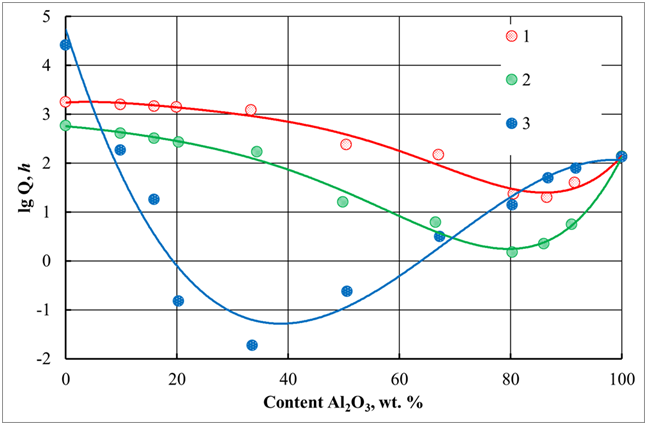
Figure 11 The dependence of the gelation time (Q, h) for a mixture of flint and alumina sols on the ratio of components and the pH of the starting silica sol.
Data were obtained at the following pH values: 1 - 1.0; 2 to 2.8; 3 - 8.0
However, in a wide pH range, the position of the minimum point on the lifetime graphs of mixed aluminum-silica sols varies quite significantly as a function of component ratio (Figure 12). A particularly significant shift of the minimum, to the side with low aluminum content in the mixed system, was observed in the alkaline region. In all cases, the stability of individual components is higher than the combined systems. The interaction of oppositely charged dispersed systems differed significantly in speed from other cases (Figure 11, curve 3); it resembled the coagulation of an alumina sol under the action of an alkaline agent. Therefore, the question arises about the nature of coagulation. In this case, two mechanisms are possible for this process to occur. The first version of this mechanism is the home coagulation of an individual dispersion under the action of an additional electrolyte introduced into the system with the second component. The second option is heterocoagulation, that is, the direct interaction of particles of individual dispersed systems.
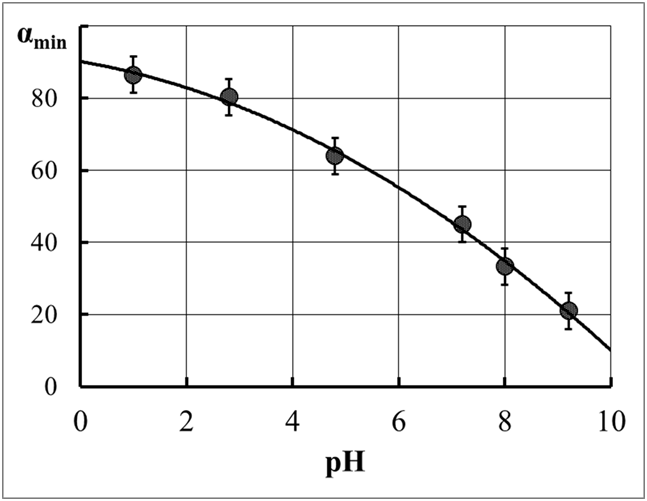
Figure 12 The position of the minimum point on the dependencies of the aluminosilicate dispersion lifetime on the pH of the initial active silicic acid.
αmin is the Al2O3 content in the aluminosilicate dispersion.
The following experiment was conducted to solve this problem. Individual model dispersed systems were prepared, active silicic acid with pH=8.0 (3 wt.% SiO2) and a suspension of aluminum hydroxide with pH = 3.6 (3 wt.% Al2O3) were mixed in a 1:1 ratio. For comparison, two systems were created that simulates the process of home coagulation under the influence of an electrolyte background:
In all cases, the concentration of the dispersed phase was 3%. The lifetime of the mixed aluminosilicate system was 10–20 min. The stability of individual model systems was significantly higher, which indicates the predominant heterocoagulation nature of the course of the coagulation process in a mixed system. This conclusion raised some doubts in the case of a significant predominance of SiO2 in the system (pH=8.0). At the first moment, when dispersions are mixed in the Al2O3:SiO2 ratios from 1:6 to 1:14, local gelation occurs, and after a certain period, gelation in the mixed system in the entire volume is observed. A mixed aluminosilicate system with a ratio of 1:13 was studied in more detail. The precipitate was separated in a centrifuge for 30 minutes (W= 10,000 rpm). The centrifuge was analyzed for SiO2 and Al2O3. The content of silicon oxide was determined by the gravimetric method (precipitated with HCl). The alumina content was determined by the titrimetric method. The relative decrease in the content of aluminum and silicon oxides was 43 and 22%, respectively. Therefore, in this case, both dispersions participate in the interaction. This fact means that the process has a heterocoagulation character.
Suspension of aluminum hydroxide has an essential specific property, which consists of the almost complete absence of the possibility of changing the charge of the particles of the starting aluminum hydroxide by adjusting the pH. We can influence its stability only during its synthesis by changing the content of anions in the system. Thus, the study of the influence of these factors on the properties of a complex aluminosilicate dispersion makes it possible to control its stability, uniformity and ensure the process along a heterocoagulation path. This fact is most important in the synthesis and use of composite flocculants-coagulants.
Natural water characterization
Most of the rivers of Russia are low-mud waters of the hydro-carbonate class of the calcium group and are distinguished by color, alkalinity, and hardness.23,37 During floods, in most rivers, turbidity and color increase, and hardness and alkalinity decrease. In winter, the water temperature drops.Chroma is one of the leading standardized indicators of the quality of purified water. This situation is because the chlorination of colored water can form highly toxic organochlorine compounds. Therefore, the study of the coagulating ability of the coagulants ASFC and ISFC was carried out on natural river waters with low turbidity of their three rivers: Orsha, Volga, and Tvertsa. These waters differ in terms of color, and their characteristics are presented in Table 4. As follows from Table 1, the river waters of the Volga and Tvertsa belong to waters with low turbidity and an average level of color, and the river water of Orsha refers to waters with a high level of color.
|
Indicator |
|
Volga |
Tvertsa |
Orsha |
|
pH |
|
7.75 |
7.78 |
7.48 |
|
Alkalinity |
mEq/L |
2.15 |
1.9 |
2.4 |
|
Turbidity |
mg/L |
10 |
12 |
25.6 |
|
Chromaticity * |
degrees |
85 |
102 |
288 |
Table 4 Characteristics of river waters
* Chromaticity was measured in degrees chrome-cobalt scale.
Comparison of the coagulating ability of coagulants in water purification with high chromaticity Orsha river
The results of the effectiveness of reducing turbidity (optical density D540) and color (optical density D400) of Orsha water depending on the dose of coagulants are presented in Figures 13-15. As follows from the data obtained for all coagulants, the dose-dependence curves of turbidity pass through a maximum, which indicates the formation of tiny and poorly precipitated flakes at these doses. The optimal dosages of aluminum-containing coagulants ASFC and AS, and iron-containing coagulants ISFC and I.S. (iron sulfate), at which there are a maximum and almost the same decrease in turbidity and color, are equal to 25 mg/l (for metal oxide). However, aluminum-containing coagulants better reduce turbidity and color of water than iron-containing coagulants, which also require additional alkalization of the purified water. When using ASFC and AS for water purification, the Orsha river does not need to adjust the pH of the treated water, which monotonously and equally decreases with increasing doses of both ASFC and AS, as shown in Figure 12. As can be seen from the presented dependences, the coagulants FSFC and iron sulfate give a profound effect of discoloration of the water of the Orsha River, which has high color. This fact is most likely due to the formation of polymer carboxylate chelate complexes because of the chemical interaction of iron ions with humic acids, or the formation of colloidal iron compounds. All these compounds have high color, which significantly affects the color performance of samples of purified water. A typical humic substance is a mixture of many molecules containing aromatic nuclei with phenolic and carbon substituents linked together. The functional groups that contribute most to the surface charge and reactivity of humic substances are phenolic and carboxyl groups. Also, humic acids contain many components in the form of derivatives of quinone, phenol, catechol, and sugar fragments.65 Another essential characteristic is charge density. Molecules can form a supramolecular structure held together by non-covalent forces, such as the van der Waals force, π-π, and CH-π bonds.66 The presence of carboxylate and phenolate groups gives humic acids the ability to form complexes with ions such as Mg2+, Ca2+, Fe2+, and Fe3+. Many humic acids have two or more of these groups arranged in such a way as to ensure the formation of chelate complexes.25 To intensify the purification of water by turbidity and color, the cationic flocculant Praestol 650 was used (Figures 16&17), which is used at many water treatment plants to increase the deposition rate of coagulated contaminants in combination with aluminum sulfate.67

Figure 13 Dependence of turbidity (D540) of coagulated and standing water Orsha river using aluminum-silicon flocculant-coagulant (ASFC) and aluminum sulfate (AS).
V - a dose of coagulant, mg/l.
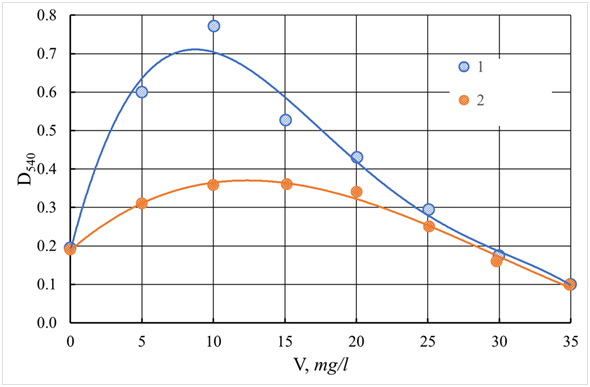
Figure 14 The dependence of the turbidity (D540) of coagulated and standing water Orsha river using ASFC (1, pH = 9.4) and iron (II) sulfate (2, pH = 9.2). V - a dose of coagulant, mg/l.
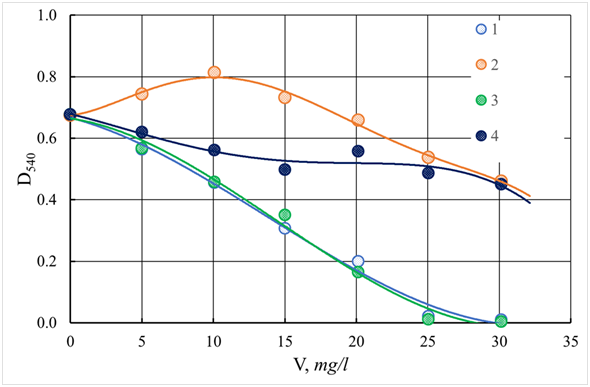
Figure 15 The dependence of the color (D400) of the coagulated and standing water Orsha river using ASFC (1), ISFC (2, pH = 9.4), Aluminum sulfate (3), and iron (II) sulfate (4, pH = 9.2). V - a dose of coagulant, mg/l.
Figure 16 The dependence of the turbidity (D540) of coagulated and standing water Orsha river with the use of ASFC and ISFC (20mg / l) together with the flocculant Praestol 650 of its dose.
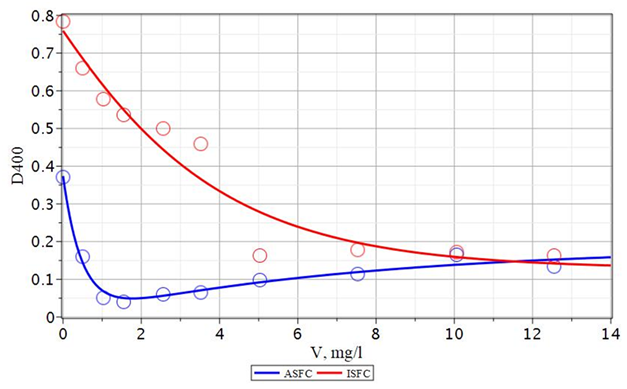
Figure 17 The color dependence (D400) of coagulated and standing water Orsha river with the use of ASFC and ISFC (20 mg/l) together with the flocculant Praestol 650 of its dose.
The dependencies shown in Figures 16& 17 are involved with an extremum. Based on this, these dependencies add up because of two mutually different processes, characterized by individual equilibrium states at a specific concentration of reacting substances. The obtained dependencies are well described by equation (21):
(21)
The first term of equation (21) is responsible for the behavior of the left falling part of the curve shown in Figures 16&17. The second term of this equation ensures the growth of the presented dependences at high doses of reagents. The ratio of the constants in this equation provides the appearance of an extremum. It can be assumed that the first term of equation (21) describes the formation and precipitation of the solid phase of mixed hydroxides, and the second may be responsible for their partial peptization and formation of a colloidal solution. Since this mechanism is valid for both ASFC and ISFC, it is most likely quite fundamental and is explained by the general laws of behavior of colloidal dispersions. As follows from Figures 16&17, the optimal dose of Praestol 650 is 1.9 ± 0.2 mg/L for ASFC, and 1.5 ± 0.2 mg/L for ISFC. Turbidity dependence (D540) of purified water Orsha from the coagulant dose with the coagulant dose and the additional flocculant Praestol-650, equal to 20: 1, is shown in Figures 18&19.
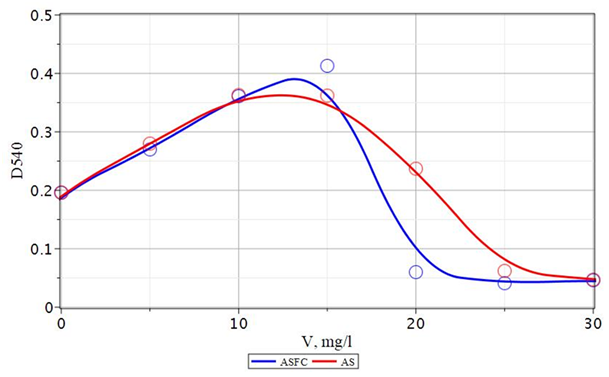
Figure 18 The dependence of the turbidity (D540) of the purified water of the Orsha River on the dose of the coagulant when the dose ratio of the coagulant and the additional flocculant is 20: 1.
ASFC - aluminum-silicon flocculant coagulant; AS - aluminum sulfate.
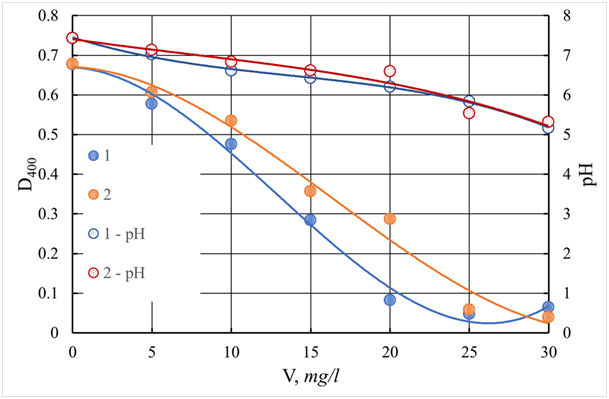
Figure 19 The dependence of the color (D400) and the pH of the purified water of the Orsha River on the dose of coagulant with the ratio of coagulant and additional flocculant equal to 20:1
At this concentration, a maximum reduction in turbidity and color of the treated water is achieved. Also, to make the effect of the corresponding ASFC, for ISFC, a significant amount of Praestol 650, equal to 12 mg/L, must be added. The observed difference in the ASFC and ISFC behavior under the action of Praestol 650 additive is due to the difference in the structure of the formed iron and aluminum hydroxides. So when using a more effective composite coagulant ASFC than ISFC, the turbidity of the water decreases from 19.3 mg/l without an additional flocculant to 8 mg/l with a flocculant, and the color decreases from 161 degrees without flocculant to 25.5 degrees with the addition of flocculant Praestol 650.68
Comparison of the action of the composite coagulant AKPK and aluminum sulfate when used in conjunction with the flocculant Praestol 650 to purify the water of the Orsha River (Figures 18&19), shows that AKPK is much more useful than aluminum sulfate. ACFC reduces turbidity and color even with a lower dose of reagents (20 ± 1 mg/l). When using aluminum sulfate, higher doses of the reagent (25.0 ± 1.3 mg/l) are required to obtain the same quality of purified water by turbidity (78 mg/l) and color (25 ÷ 31 mg/l). Thus, for the purification of water with high color and low turbidity, such as the river water of the Orsha River, it is more efficient to use the composite coagulant ASFC together with Praestol 650 than ISFC and aluminum sulfate.
Comparison of the coagulating ability of coagulants in the treatment of the Volga River with medium color
The experimental results obtained by the effectiveness of the use of composite coagulants ASFC, ISFC, and basic coagulants - aluminum sulfates and ferrous iron for the purification of Volga water are presented in Figures 20-25. As follows from the data obtained, to reduce the color of the source water of the Volga River in comparison with the water of the Orsha River, lower doses of coagulants are required to achieve optimal conditions. In this case, the maximum effect of reducing turbidity and color is achieved. If, for purification of the water of the Orsha River, the doses of coagulants were 20–25 mg/L, then for the Volga water, it was 12.5–15 mg/L. The appearance of the dependencies of turbidity and color on the dose has not changed. The dependence of the turbidity of purified water on the dose of the coagulant passes through a maximum (Figure 20). This behavior indicates a low deposition rate of coagulated contaminants and the need for additional water filtration. It is also possible to use additional flocculants to enlarge coagulated pollutants and increase the speed of their deposition during sedimentation.
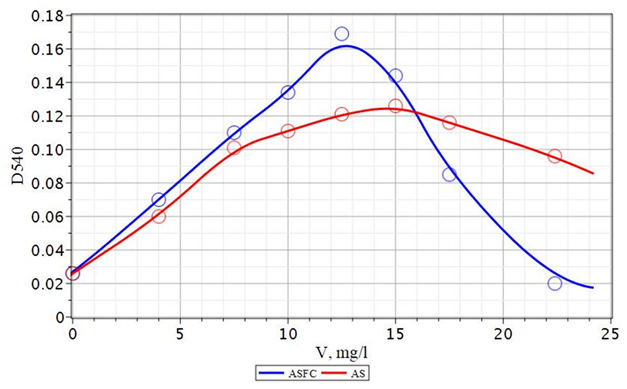
Figure 20 Dependence of turbidity (D540) of coagulated and standing water p. Volga with the use of ASFC and aluminum sulfate (AS). V - a dose of coagulant, mg/l.
The color of the purified water monotonously decreases with an increasing dose of ASFC and reaches a minimum (8.5 degrees) at a dose of 17.5 mg/l (Figure 21). The color value of purified water, equal to 19 degrees, which is lower than the normalized value (20 degrees), is achieved with a dose of ASFC 12.5 mg/l, at which aluminum sulfate provides a residual color of 23.3 degrees (Figure 20). The use of coagulants in combination with the flocculant Praestol 650 is accompanied by an increase in the effect of water purification by turbidity, both when using ASFC and aluminum sulfate. The optimal dose of flocculant is 0.5 mg/l, with a dose of coagulants of 15 mg/l (Figures 23). The residual turbidity is 2.68 mg/l when using ASFC and 1.34 mg/lwhen coagulating with aluminum sulfate, whereas without flocculant, the turbidity of the standing water is 16.7-19.4 mg/l. In the case of the use of ASFC, to achieve turbidity of 1.34 mg/l, the dose of the flocculant should be increased to 0.75 mg/l(Figure 22). The color of the Volga water is better removed when using ASFC than aluminum sulfate, both with the independent use of coagulants and in combination with the flocculant Praestol 650 (Figures 21&23).
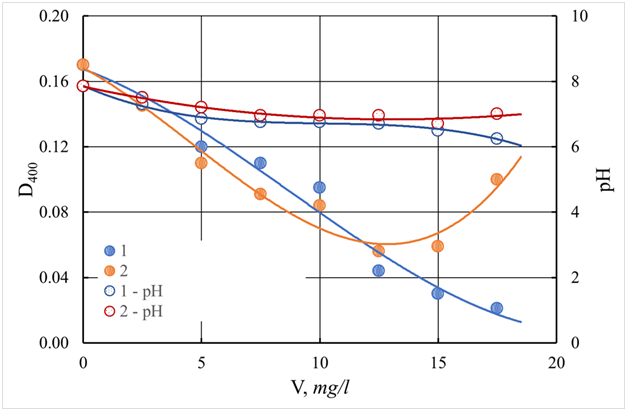
Figure 21 Dependence of color (D400) and pH of the purified water of the Volga River on the dose of coagulant. The ratio of coagulant and additional flocculant equals 20:1. V - a dose of coagulant, mg/l.
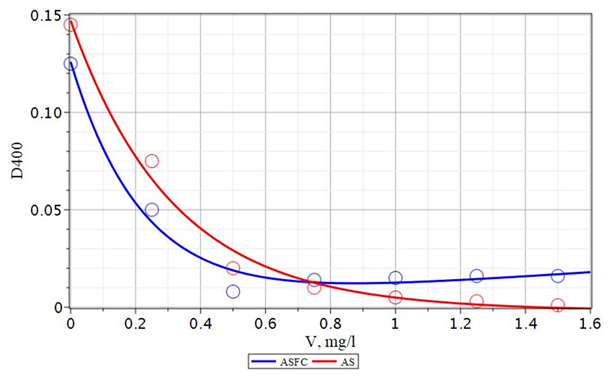
Figure 22 Dependence of the D540 turbidity of the protected water of the Volga River on the dose of an additional flocculant Praestol 650 at a coagulant dose of 15 mg/L. V - a dose of coagulant, mg/l.
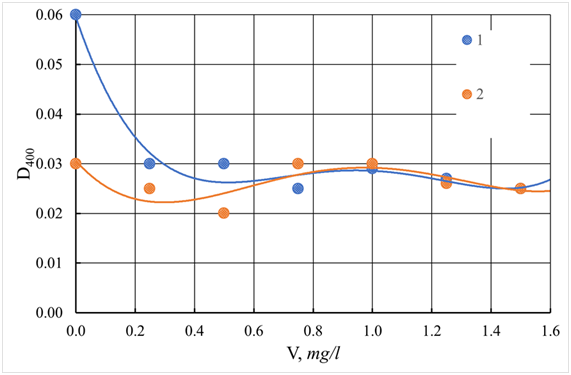
Figure 23 The dependence of the color D400 of the protected water of the Volga River on the dose of an additional flocculant Praestol 650 at a dose of coagulant 15 mg/L.
1 - ASFC; 2 - aluminum sulfate; V - a dose of coagulant, mg/l.
As follows from Figures 24&25, iron-containing coagulants increase the color and turbidity of the source water. Such effects are observed both with independent use and together with a flocculant. They are due to the formation of humate complexes with iron ions, or the creation of colloidal forms of products of hydrolysis of iron sulfate, which have high color. An increase in the pH of the purified water when using ISFC helps to reduce residual turbidity, color, and dissolved iron (Figure 26). The maximum decrease in turbidity and color occurs in the alkaline region at a pH of more than 9.0. The results presented in Figure 27 show that when cleaning Volga water, as well as Orsha water, aluminum-containing coagulants are more effective when used in combination with the cationic flocculant Praestol 650. In this case, the composite coagulant ASFC in comparison with aluminum sulfate better reduces color water, and in conjunction with the flocculant - and the turbidity of the Volga water.69

Figure 24 Dependence of the turbidity D540 of the Volga River treated water on the dose of the coagulant-flocculant ISFC.
1 - Individual ISFC. 2 - ISFC together with an additional flocculant Praestol-650, with a ratio of 20:1.
V - a dose of coagulant, mg/l.
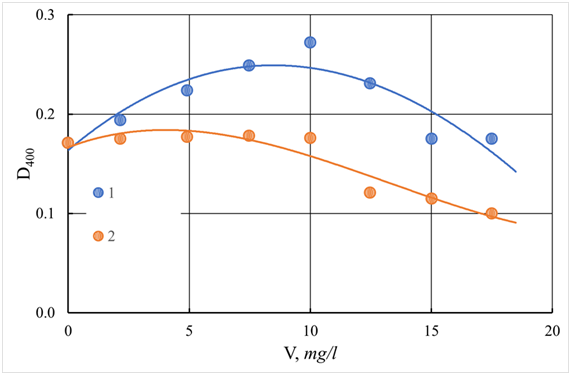
Figure 25 The dependence of the chromaticityD400of the Volga River treated water on the dose of the coagulant-flocculant ISFC.
1 - Individual ISFC. 2 - ISFC together with an additional flocculant Praestol-650, with a ratio of 20:1
V - a dose of coagulant, mg/l.
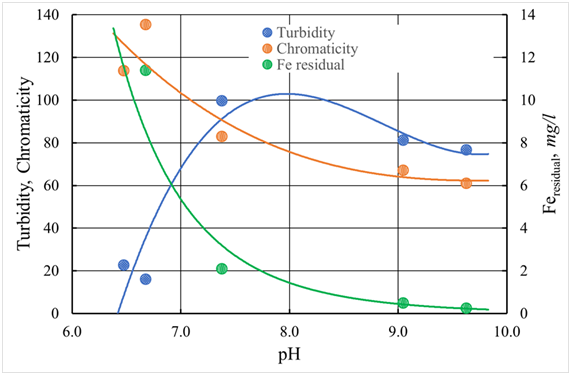
Figure 26 The pH dependence of the coagulated and defended water of the Volga river with the use of ISFС with a dose of 15 mg/l at a temperature of 10 °C.

Figure 27 The effectiveness of reducing the color and turbidity of the river p. Volga coagulants (15 mg/l) and together with the additional flocculant Praestol-650 (0.75 mg/l).
Comparison of the coagulating ability of coagulants in the treatment of water of medium color of the Tvertsa River
The surface waters of the Tvertsa river are similar in chemical composition to the Volga water (Table 2); therefore, it can be assumed that the effectiveness of the use of coagulants for cleaning the Tvertsa and Volga rivers will be close. The results, presented in Figures 28-31, confirm this assumption even though the temperature of the treated water of the Tvertsa River was 10 °C, which is two times lower than the Volga water (20 °C). The optimal doses of aluminum sulfate and ASFC, at which the color is removed as much as possible, are the same and equal to 12.5 mg/l (Figure 29). The residual color is 21-23 degrees. Turbidity curves also pass through a maximum (Figure 28), as in the coagulation treatment of Volga water. When using ISFC, cleaning efficiency depends on the pH of the water. The greatest decrease in turbidity and especially the color of the purified water of the Tvertsa River is observed at pH = 9.3 and the new introduction of an additional flocculant Praestol 650 with the ratio of coagulant: flocculant = 20 (Figure 29). The use of a flocculant with a dose of 0.35-0.5mg/l increases the cleaning effect by turbidity during sedimentation to a greater extent than by color (Figures 31&32).
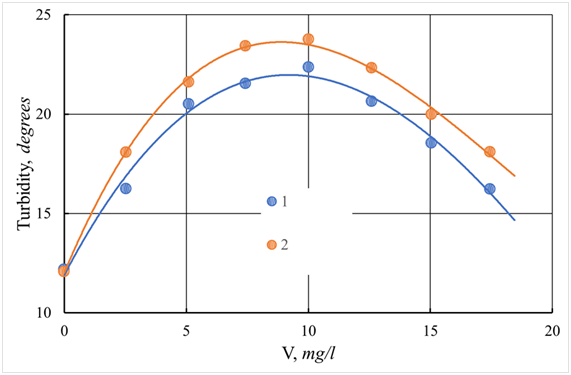
Figure 28 Dependence of turbidity of the standing water of the river Tvertsa from a dose of coagulant.
1 - ASFC; 2 - aluminum sulfate; V - dose of coagulant, mg/l.
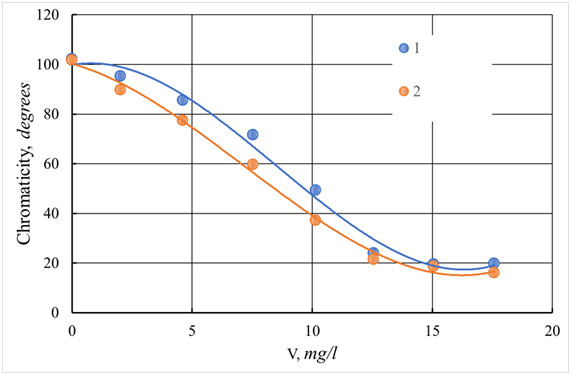
Figure 29 The dependence of the color of the standing water of the river Tvertsa from a dose of a coagulant.
1 - ASFC; 2 - aluminum sulfate; V - dose of coagulant, mg/l.

Figure 30A The dependence of the turbidity of the coagulated water of the Tvertsa River on the dose of ISFC at different pH of the treated water.

Figure 30B Dependence of the color of the coagulated and defended water of the Tvertsa River on the dose of ISFC at different pH of the treated water.
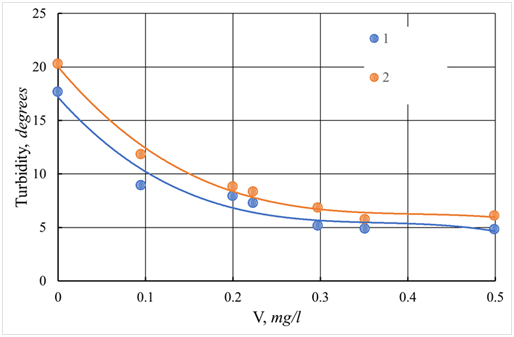
Figure 31 Dependence of the turbidity of the protected water of the Tvertsa River on the dose of the additional flocculant Praestol-650 at a dose of coagulant 12.5 mg/l.
1 - ASFC; 2 - aluminum sulfate; V - dose of coagulant, mg/l.
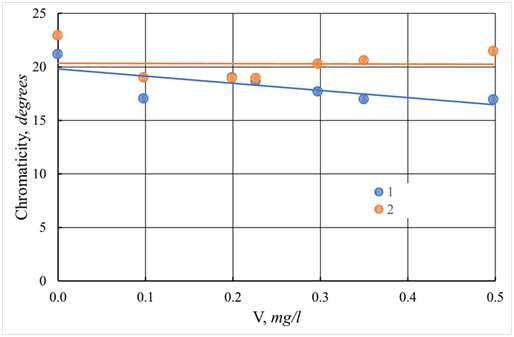
Figure 32 Dependence of the turbidity of the protected water of the Tvertsa River on the dose of the additional flocculant Praestol-650 at a dose of coagulant 12.5 mg/l.
1 - ASFC; 2 - aluminum sulfate; V - dose of coagulant, mg/l.
The content of residual aluminum in water is minimal at the optimal dose of coagulants (Figure 33) but is slightly higher when using ASFC than aluminum sulfate. However, in both cases, it is five or more times lower than average (0.5 mg/l). Thus, composite coagulants ASFC and ISFC and basic coagulants-sulfates of aluminum and iron exhibit similar coagulating properties in the purification of natural waters of various water sources.
It has been experimentally shown that the composite flocculant ASFC is more effective than aluminum sulfate in the treatment of slightly turbid waters with high and medium color in terms of color at equal or lower doses. The simultaneous achievement of a high rate of water purification by sedimentation by color and turbidity is achieved with the use of ASFC together with the flocculant Praestol 650 with a coagulant/flocculant ratio of 20:1. Composite coagulant ISFC and iron sulfate do not provide the required color purification effect of natural waters even in an alkaline environment, which makes their use in existing water treatment schemes inappropriate. Their purpose is possible in technologies for purifying natural waters with deep color, provided that unusual, more stringent coagulation conditions are created, and ferrous iron is converted to ferric, as well as for wastewater treatment from salts of heavy metals.
The authors express their gratitude to the head of the laboratory of reagent water treatment methods Integrated research and development and technological institute of water supply, sewage, hydraulic structures, and engineering hydrogeology OJSC «NIIVODGEO», to Doctor of Chemistry Gandurina L.V., for help in testing composite flocculants-coagulants.
Authors declare no conflict of interest exists.
None.

©2020 Kudryavtsev, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.