International Journal of
eISSN: 2576-4454


Research Article Volume 6 Issue 4
1Graduate Student in Environmental and Sanitary Engineering - State University of Rio de Janeiro, Brazil
2Graduate in Environmental and Sanitary Engineering - State University of Rio de Janeiro, Brazil
3Doctoral student in Environmental Engineering - State University of Rio de Janeiro, Brazil
4Associate Professor in the Department of Sanitary and Environmental Engineering of the State University of Rio de Janeiro, Brazil
5Adjunct Professor at the Department of Sanitary and Environmental Engineering of the State University of Rio de Janeiro, Brazil
6Rua São Francisco Xavier, 524, Maracanã, Rio de Janeiro – RJ, Brazil - CEP 20550-900, Brazil
Correspondence: Alfredo Akira Ohnuma Junior, Adjunct Professor at the Department of Sanitary and Environmental Engineering of the State University of Rio de Janeiro, Brazil, Tel +55 (21) 99591-7373
Received: June 30, 2022 | Published: July 11, 2022
Citation: de Almeida JCA, de Souza CM, Alves LD, et al. The effects of seasonality on the quality of rainfall water in the city of rio de Janeiro. Int J Hydro. 2022;6(4):118-128. DOI: 10.15406/ijh.2022.06.00316
Critical periods and abnormal rainfall events have been observed in the South-East of Brazil, and this has affected both the amount and quality of the volume of water in the water supply systems. This study seeks to assess the influence of seasonal factors on the quality of rainfall at a water treatment plant, located in the city of Rio de Janeiro - RJ. The methodology involved collecting rainfall samples between January 2017 and December 2018, at four specific points in the system: first flush 1 (FF1), first flush 2 (FF2), void volume (VV) and reservoir (RR). The parameters were analyzed statistically and followed seasonal patterns for each of the seasons of the year, and included: pH, turbidity, electric conductivity, total of alkalinity and chloride. The results suggest that there is an urgent need to dispose of the initial volume stored in the devices of the first flush. The water contained in the tank was of a satisfactory standard for non-drinkable purposes despite the effects of seasonality on the quality of the rainfall water. A multivariate statistical correlation analysis made it possible to assess the influence of the seasons of the year on the quality of pluvial water.
Keywords: water quality, urban water, water resources, statistical treatment
Environmental significance statement
Rainfall harvesting systems operate in accordance with the seasonality of the affluent volumes of rainfall. These are able to reduce the overload of the urban microdrainage system and mitigate the effects of the high demand for water in cities. In light of this, it is essential to assess the operational conditions of the rainwater harvesting systems with regard to the rainfall patterns, especially by conducting collections and laboratory analyses.
Since 2012, different regions of Brazil have undergone prolonged dry periods and droughts at certain times of the year, which has restricted the amount of water that can be offered from the public supply systems, especially those that depend on storage and the volume of water in reservoirs.1 In a search for a way to reduce the effects of this scarcity, water harvesting catchment has arisen as an alternative measure in regions that experience rainy seasons and a means of maintaining the tanks and supply of water from rainfall to their maximum capacity (GOMES et al., 2008; BENETTI, 2019).
In Brazil, it is estimated that there has been a rise in the withdrawal of water of 80% in the last two decades with a predicted equivalent increase of 24% by 2030. In 2017, irrigation work led to the greatest withdrawal of water with an annual average of 52%, followed by the urban supply (23.8%), industrial use (9.1%), supply for animals (8%), thermoelectric power (3.8%), rural supply (1.7%) and the mining industry (1.6%).1
Rainwater can be regarded as an alternative source of clean water when it is available and can be collected and stored in systems for harnessing rainwater2 and its quality has been widely discussed throughout the world with regard to whether its use can be characterized as suitable for drinkable or non-drinkable purposes.3–6
Atmospheric air pollutant dispersion is spread by rainwater and deposited on the earth´s surface. As a result, there is a cleaning of the surface water in the catchment, with a removal of the substances present in the area of contact. These factors affect, for example, the quality of the rainwater in the storage systems that is captured from rooftops.7
There is still no consensus, or consistent conclusions in the international literature, about the quality of rainwater in regions with similar features. Some studies argue that the quality of rainwater is acceptable for a wide range of uses,8 while others have detected the presence of a number of contaminants in the samples which does not make its use feasible.5
Although each year there has been an average increase, (ranging from 13% to 20%) in the number of publications in scientific periodicals regarding the issue of harnessing rainwater and assessing the quality of rainfall, there have been few studies explaining the role of seasonal factors, through wet and dry deposition at different sampling points of the catchment area and the storage of water in rainfall systems. Zhang et al.9 and Souza et al.10 provide the seasonal results obtained from an analysis of the parameters of the quality of rainfall collected at a single point of the system and that have different positive features. The objective of this study is to characterize the rainwater on the basis of samples collected at 4 different points of a catchment and storage system of rainfall located in the city of Rio de Janeiro. The study involved carrying out a statistical survey of year-on-year seasonality based on a correlation matrix and an analysis of the key components of the physico-chemical parameters - pH, turbidity, electric conductivity, total of alkalinity and chloride.
Area of study in the city of Rio de Janeiro
The rainwater catchment and storage system (SAP), installed at the State University of Rio de Janeiro (UERJ) for harnessing rainwater from the covered garage of official vehicles - coordinates 22° 54' 43.68" S and 43° 14' 6.60" W, is located in the district of Maracanã, Rio de Janeiro – RJ (Figure 1).
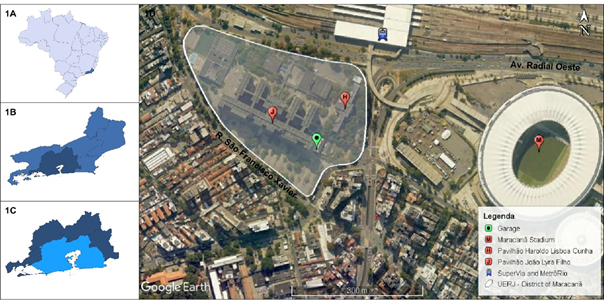
Figure 1 Location of the catchment and storage system of rainfall, Rio de Janeiro - RJ. 1A: State of Rio de Janeiro. 1B: Metropolitan Region of Rio de Janeiro. 1C: Micro-region of Rio de Janeiro. 1D: UERJ and garage coverage with a catchment area of 30 m² – District of Maracanã. Source: IBGE [Brazilian Institute of Geography and Statistics, 2021] and GOOGLE EARTH (2021).
The district of Maracanã forms a part of the Vila Isabel Administrative Region (RA IX), together with the districts of Andaraí, Grajaú and Vila Isabel. It comprises a population of 25,256 inhabitants (2010) and is situated in an urban area of 166.73 ha (2018), with asphalt streets and rivers formed into canals. It is crowded with people, which shows the widespread use and occupations of the diversified land (residential dwellings, businesses, technical colleges, universities, hospitals and entertainment facilities). The railway and metro stations carry a daily average of 9,000 passengers (2017) for each system of transport.11 The locality chosen for this study has two direct means of access to the region, namely a) Rua São Francisco Xavier with an average flow of traffic on work days of 20,909 vehicles - close to Maracanã Square, in the direction of Tijuca and b) Radial Oeste Avenue with 56,021 vehicles - close to the Maracanã Stadium, in the direction of Méier.12
The catchment area and storage capacity of the rainwater harvesting system (SAP)
The rainwater harvesting system (SAP) has been installed in a catchment area of 30 m² in the garage roof coverage of the vehicles belonging to the institution. It is formed of fiber cement building material, gutters, vertical and horizontal PVC conductors, connections, a separator system of solids (SS), a separator system for a first outlet of discharge or first flush (primary - FF1 and secondary - FF2), an overflow system, a water level gauge, a tank lined in polyethylene and collection points. The SAP also has a primary treatment system called Chove Chuva, [downpour] formed of limestone and chlorine tablets for the correction of the pH and disinfecting of the rainwater.
The quality of the volume stored in the tank is directly affected by the initial cleaning of the rooftop carried out by the separation system of the first outlet of discharge or first flush (FF). The SAP comprises the FF1 and FF2, and has a total storage capacity of 1.0 mm, divided into two equal parts of 0.5 mm. After the separation of the first FF1 and FF2 volumes, the water is channelled to the Chove Chuva treatment system. In this system, the water is tranferred to a stainless steel inox filter for the elimination of multi-dimensional residues. After this, the rainwater flows over the limestone for the adjustment of the pH and the affluent volume of the system is disinfected by means of chlorine tablets with the aim of eliminating any possible pathogenic microorganisms before being stored in the tank.
The tank lined with polyethylene is used to store 1000 liters of rainwater or, in effect, an accumulated level of rainfall of 33 mm.
The SAP has 4 collection points (Figure 2): primary first flush (FF1), secondary first flush (FF2), void volume (VV) and reservoir (RR).
Period for the collection of samples
The samples were basically collected once a month in the years 2017 and 2018, with the exception of a) March and August 2017 when there were two collections each month, b) the lack of a collection in July 2017 and c) two collections each month in January and March 2018. The volumes were stored in polythene plastic bags with a capacity of one liter. The National Standard Operating Procedures for the Collection and Preservation of Samples was used for the cleaning of the containers, the types of vessels used, storage and the preservation of the samples.13
Following the guidelines of the Institute of Astronomy, Geophysics and Atmospheric Sciences (IAG), the dates for the beginning of the seasons of the year were as follows: Summer – 21st December; Fall – 20th March; Winter – 21st June; and Spring – 22nd September.14 The samples collected between January 2017 and December 2018, covered a total of 27 campaigns, namely: 10 in Summer, 5 in Fall, 7 in Winter and 5 in Spring. These campaigns resulted in a total of 95 samples collected in a combined way from the points of the SAP.
In 2017, there was no volume of rainfall in the September collection for the VV and RR points and the collections carried out in the months of March, August and October did not record any volume for the RR point, making a total of 5 campaigns. During 2018, no rainfall was stored for the VV and RR points in the collections of February and March, and in July and October for the RR point, making a total of 8 campaigns.
Local rainfall patterns
Information about local rainfall patterns can be obtained from the nº 4 Station of the Tijuca Early Warning System based at the Rio de Janeiro City Council (ALERTA RIO, 2021). Although it represents features that are typical of a humid tropical climate with heavy rainfall between October and March, the pluviometric data between 2017 and 2018 revealed a disproportionate amount of rainfall collected in the region of Tijuca. The year 2017 showed a total annual rainfall of 863 mm, while in 2018 about 1271 mm was recorded, which means it can be characterized as a more humid hydrological period than the previous year (Figure 3).
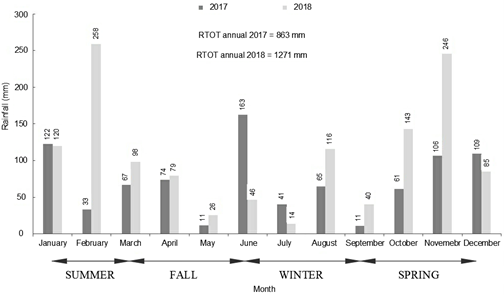
Figure 3 Monthly and total annual rainfall in 2017 and 2018 at the Tijuca station. RTOT: Total Rainfall. Source: Early Warning System, Rio de Janeiro City Council (ALERTA RIO, 2021).
In 2017, the pattern of rainfall in terms of seasonality was as follows: 298 mm in Summer, 237 mm in Fall, 143 mm in Winter and 186 mm in Spring. In 2018, these data corresponded to 480 mm in Summer, 188 mm in Fall, 165 mm in Winter and 438 mm in Spring. With the exception of Fall, the annual seasons of 2018 represented a more humid period than the previous year, especially at the end of Spring, when there was a rise in the pluviometric index for the month of November 2018. These rates of rainfall led to annual seasonal averages of 389 mm in Summer, 212 mm in Fall, 154 mm in Winter and 312 mm in Spring.
An analysis of water quality through the use of physico-chemical parameters
The samples analyzed ex situ, at the Laboratory of Sanitary Engineering (LES) of UERJ for characterizing the stored volumes, included the following physico-chemical parameters: pH, turbidity, electric condutivity, total alkalinity and chloride. They made use of certain devices such as: TECNOPON turbidimeter model TB-1000, QUIMIS pH meter model Q400AS and QUIMIS benchtop conductivity meter Q405M.
All the analyses were based on the Standard Methods for the Examination of Water and Wastewater (Table 1).29
Parameter |
Method (APHA, 2012) |
Total Alkalinity |
2320 B: Titration Method |
Chloride |
4500 – Cl– B: Argentometric Method |
Electric Conductivity |
2510 B: Laboratory Method |
pH |
4500 – H+ B: Electrometric Method |
Turbidity |
2130 B: Nephelometric Method |
Table 1 Analytical methods of physico-chemical parameters used for the samples of rainwater
Statistical analysis of the rainwater samples
The data collected were treated statistically in accordance with the seasons, by means of boxplot graphs, a correlation matrix and a principal components analysis (PCA).
The boxplot is represented by the parameter in each point of the collection system from seasonal variations throughout the different seasons of the year. This graph displays the lower and upper quartile, where they form the graphic box in which 50% of the results are concentrated. In addition, the minimum and maximum limits, the average and the outliers are shown. When applicable, each boxplot complies with current legislation. The total number of results analyzed include 95 samples formed of 27 campaigns spread over 2 hydrological periods between 2017 and 2018.
The correlation matrix of the parameters is divided into the seasons of the year and carried out by means of ellipses, variations of color and the correlation coefficient, whereas the PCA method is displayed in a unit circle that contain two of the principal components showing the physico-chemical parameters for the season of the year.
Both the correlation matrix and the principal components analysis required conducting 13 campaigns which corresponded to a total of 52 samples. Only the campaigns in which all the parameters analyzed showed results at every point were selected and this allowed the data from the samples to be correlated. On account of this, 14 campaigns failed to meet this requirement.
The graphs were obtained through the use of R Language.15
Legislation
As a means of standardizing the characterization of the rainfall collected from the harvesting rainwater system (SAP), the NBR [Brazilian Regulatory Standards] 15.527/2019 (ABNT, 2019) was used together with the Consolidation Ordinances nº 888/2021 (MS, 2021) (Table 2). Owing to the lack of legislation which could allow the parameters to be determined for the harnessing of rainwater, the ordinance was adopted with the aim of setting out the guidelines for analyzing how the rainwater could be used for non-drinkable purposes.
Parameter |
NBR 15.527/2019 |
ORDINANCE Nº 888/2021 (APPENDIX 20) |
Chloride (mg. L-1 Cl-) |
- |
< 250 |
pH |
6 – 9 |
6 – 9,5 (1) |
Turbidity (NTU) |
< 5 (2) |
< 5 (3) |
Table 2 Limits set for the standardization of the quality of the rainwater. Source: the author herself
(1) For the supply system;
(2) < 5 NTU: maximum value permitted;
(3) Limit referring to the standard of organoleptic testing for drinkability
The hydrological year is divided into Summer, Fall, Winter and Spring with each season lasting for approximately three months and showing the distinct climatic features of the region used for this study.
Summer is a season noted for an increase of temperature caused by greater solar radiation, and a rise in the occurrence of convective and orographic rainfall in a region of strong pluviometric intensity and short duration. The Fall is a tranisitonal period which marks the end of the rainy period and the beginning of the dry period, and brings about a drop in temperature, a reduction in the amount of rain and an increase in wind speed. The Winter is the driest and coldest period as a result of a reduction in pluviometric precipitation and lower temperatures. As well as this, the low humidity leads to drier days and an increase in air pollution. The Spring can also be characterized as a transitional period which marks the end of the dry season and beginning of the rainy season, which alters the pattern of rainfall and causes a rise in local temperature.16,17
The geographical location of the rainwater harvesting system within the Maracanã district in the city of Rio de Janeiro, is leading to an alteration in the composition of rainwater as a result of features involved in rainfall events, the seasons of the year and atmospheric pollution.
Hydrogenionic potential (pH)
The results for the season of the year and the collection point are provided in Figure 4. The FF1 fluctuated between 6.5 and 9.5, with an overall average of 7.26 ± 0.71. In the case of the FF2 point, this variation ranged from 6.4 to 10.7, with an overall average equal to 7.37 ± 0.93. The VV point had a minimum value of 6.6 and maximum of 8.6 with an overall average of 7.28 ± 0.49. The data for the RR fluctuated from 6.5 to 8.2, with an overall average of 7.19 ± 0.44.
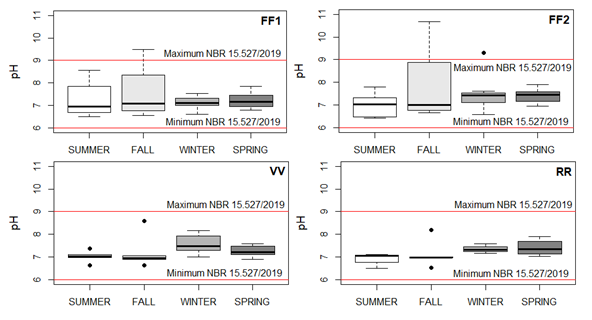
Figure 4 Boxplot of the pH at points FF1, FF2, VV and RR, corresponding to the seasons of the year. Source: the author herself.
Table 3 displays the statistical results at each collection point for the season of the year, and shows the minimum and maximum values, the average and standard deviation.
SEASON |
PT |
DATA |
SEASON |
PT |
DATA |
||||||
Min. |
Max. |
Average |
SD |
Min. |
Max. |
Average |
SD |
||||
Summer |
FF1 |
6.5 |
8.57 |
7.25 |
0.83 |
Winter |
FF1 |
6.61 |
7.52 |
7.13 |
0.3 |
FF2 |
6.42 |
7.79 |
6.99 |
0.51 |
FF2 |
6.58 |
9.31 |
7.52 |
0.86 |
||
VV |
6.63 |
7.37 |
7.03 |
0.27 |
VV |
7.01 |
8.16 |
7.56 |
0.43 |
||
RR |
6.49 |
7.1 |
6.92 |
0.29 |
RR |
7.15 |
7.58 |
7.35 |
0.22 |
||
Fall |
FF1 |
6.56 |
9.48 |
7.55 |
1.31 |
Spring |
FF1 |
6.79 |
7.84 |
7.23 |
0.42 |
FF2 |
6.65 |
1.67 |
7.83 |
1.9 |
FF2 |
6.96 |
7.91 |
7.41 |
0.37 |
||
VV |
6.63 |
8.6 |
7.22 |
0.78 |
VV |
6.9 |
7.58 |
7.26 |
0.28 |
||
RR |
6.53 |
8.19 |
7.14 |
0.62 |
RR |
7.04 |
7.9 |
7.41 |
0.38 |
Table 3 Results of the analysis of pH. Source: the author herself
PT, point; Min, minimum value; Max, maximum value; SD, standard deviation
In general terms, the averages of all the collection points can be found in the ranges of pH Alkaline. The FF2 and RR points recorded the lowest pH in Summer. The highest values were found at the FF1 points in the Fall and FF2 in Fall and Winter. In the case of all the points, the data were more widely dispersed in the Fall. The lowest average of pH was found in the Summer at the RR point. The largest averages recorded in the Fall were at the FF1 and FF2 points, in Winter at the FF2 and VV points and in Spring at the FF2 and RR points. In the case of the RR point, there was a constant growth throughout the year.
The NBR 15.527/2019 stipulates that the range of pH should be between 6 and 9. All the points reached the determined minimum value (pH=6) although they diverged from the maximum permitted value. The percentages of the samples with pH below 9 were FF1 with 95.8 %, and FF2 corresponding to 91.7 %, while VV and RR had 100% of the samples in agreement. The Consolidation Ordinance nº 888/2021, defines the range as being between 6 and 9.5. The FF2 point had 95.8 % of its results lower than 9.5, while FF1, VV and RR corresponded to 100 % of the results. The seasons which conformed to the minimum and maximum limits laid down by the standard were as follows: Summer. Winter and Spring for the FF1, Summer and Spring for FF2 and all the seasons for the VV and RR.
The rainfall obtained in a direct form from the annual total precipitation tended to be of an acidic character in urban areas. However, after the water drained off the fiber cement roofing, there was a rise in the pH, and two averages were recorded with pH £7 in the effective precipitation. In a general way, the period with most rain (the Summer) had the lowest averages of pH. The study carried out by Cunha et al.18 showed that in Summer and Spring, the pH was slightly lower than in the Fall and Winter, which reveals the dynamics of the pH of rainfall. Solar radiation gives rise to reactions of gases with the rain and thus forms acids that can reduce the pH,19 which explains why Summer is the season with the lowest averages of pH.
The rainwater harvesting system of this study uses limestone from the Chove Chuva [downpour] equipment to control the pH, before the water is stored in the tank, since it can be influenced in the alkinilization of the samples of effective precipitation at the VV and RR points.
Turbidity
The analysis of turbidity during the seasons of the year (Figure 5) resulted in a variation at the FF1 point ranging from 0.00 to 101.00 NTU, with an overall average of 19.52 ± 31.78 NTU. The fluctuation of the results at the FF2 point ranged from 0.10 to 104.00 NTU, with an overall average of 15.18 ± 29.66 NTU, while the VV point had a minimum value of 0.00 NTU and a maximum of 4.50 NTU, with an overall average of 0.71 ± 0.90 NTU. The variation of the data in the RR ranged from 0.00 to 1.50 NTU, with an overall average of 0.50 ± 0.38 NTU.

Figure 5 Boxplot of the turbidity at the FF1, FF2, VV and RR points, corresponding to the seasons of the year. Source: the author herself.
The assessment of turbidity with regard to the seasons of the year resulted in minimum and maximum values, the average and standard deviation for each point in the system (Table 4).
SEASON |
PT |
DATA |
SEASON |
PT |
DATA |
||||||
Min. |
Max. |
Average |
SD |
Min. |
Max. |
Average |
SD |
||||
Summer |
FF1 |
0.08 |
101 |
23.67 |
38.88 |
Winter |
FF1 |
0.1 |
4.5 |
1.65 |
1.47 |
FF2 |
0.1 |
102 |
20.98 |
35.69 |
FF2 |
0.27 |
6 |
1.9 |
1.91 |
||
VV |
0.23 |
4.5 |
1.14 |
1.5 |
VV |
0 |
1.44 |
0.65 |
0.52 |
||
RR |
0.28 |
1.5 |
0.67 |
0.44 |
RR |
0 |
0.98 |
0.54 |
0.5 |
||
Fall |
FF1 |
0.18 |
91 |
38.2 |
38.34 |
Spring |
FF1 |
0 |
49 |
17.06 |
22.94 |
FF2 |
0.21 |
104 |
33.66 |
42.91 |
FF2 |
0.59 |
11.5 |
3.71 |
4.66 |
||
VV |
0.23 |
1,21 |
0.51 |
0.42 |
VV |
0.1 |
0.76 |
0.4 |
0.28 |
||
RR |
0.1 |
0 |
0.36 |
0.18 |
RR |
0 |
0.8 |
0.4 |
0.43 |
Table 4 Results of the analysis of turbidity, NTU = Unit. Source: the author herself
PT, point; Min, minimum value; Max, maximum value; SD, standard deviation
The highest recorded values of turbidity were found at the FF1 and FF2 points, mainly in the Summer and Fall; however, the Winter had values lower than 7 NTU at this point. In general, the values obtained at the VV and RR points were lower than 5 NTU, which shows that the reduction of turbidity through the disposal of the initial volume, was an efficient method. In the case of the FF1 and FF2 samples, there was an increase in the average in the period from Summer to the Fall with a reduction in Winter and a rise of turbidity in Spring. Summer had a higher average at the VV and RR points, followed by Winter. The first disposal points of effective precipitation showed results with a standard deviation above the average value, especially in Summer; this meant there was a great variability in the analyzed data which reflects the high dispersal rate of pollutants during the rainy season.
The NBR 15.527/2019 fixes the absolute permitted limit of 5.0 NTU that can be used. The points of first flush (FF1 and FF2) indicated the non-compliances. FF1 had 65.4 % below 5 NTU and in the case of FF2 it was 63.0 %. All the samples of the VV and RR were within the limit that was set at 5 NTU. These results confirm that the quality of the water improved as a result of the removal of suspended solids and the effect of the initial blocking system in the treatment of the rainwater. All the seasons conformed to the standardized limits required for the VV and RR points.
The greatest rates of turbidity were found in the FF1 and FF2 points in the Summer and Fall, which suggests there was a greater concentration of suspended solids in the first waters. In Winter, which can be characterized as a period of lower precipitation, the points of first flush (FF1 and FF2) had values that were lower than the seasons with greater rainfall. In all the seasons, there proved to be a greater degree of turbidity at the FF1 and FF2 points, to an extent that explains the need to dispose of the first volume of effective precipitation. After the storage of this initial flow, together with the primary filtration installed in the system, there was found to be an effective reduction of the turbidity at the VV and RR points. Since the greatest results of turbidity are located at the FF1 and FF2 points in the rainy period, it can be seen that the increase in the volume of drained water leads to a greater shifting of sediment along the pathway followed by the drainage system.
Electric conductivity
The annual seasonal analysis of conductivity (Figure 6) resulted in a fluctuation at the FF1 point, ranging from 53.1 to 173.4 µS.cm-1, with an overall average of 99.06 ± 33.57 µS.cm-1. The variation in the results at the FF2 point was between 56.5 and 166.4 µS.cm-1, with an overall average of 97.30 ± 31.49 µS.cm-1, whereas the VV point had a minimum value of 44.1 µS.cm-1 and maximum of 132.2 µS.cm-1, and an overall average of 81.69 ± 29.05 µS.cm-1. The data at the RR point fluctuated between 48.2 and 128.0 µS.cm-1, and the overall average was 75.10 ± 26,60 µS.cm-1. Table 5 shows the minimum and maximum values. Average and standard deviation of the results for electric conductivity regarding each point of the system for the seasons of the year.
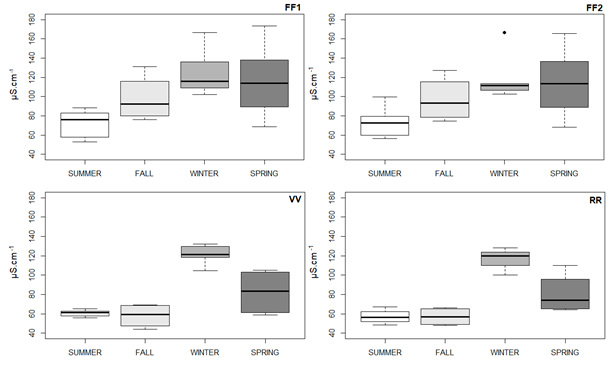
Figure 6 Boxplot showing conductivity at the points of FF1, FF2, VV and RR, corresponding to the seasons of the year. Source: the author herself.
SEASON |
PT |
DATA |
SEASON |
PT |
DATA |
||||||
Min. |
Max. |
Average |
SD |
Min. |
Max. |
Average |
SP |
||||
Summer |
FF1 |
53.1 |
88.3 |
71.8 |
13.7 |
Winter |
FF1 |
102.3 |
166.4 |
125.9 |
25.9 |
FF2 |
56.4 |
99.6 |
72.5 |
14.5 |
FF2 |
102.7 |
166.4 |
120 |
26.3 |
||
VV |
55.9 |
65.1 |
60.6 |
3.8 |
VV |
104.3 |
132.2 |
121.1 |
10.9 |
||
RR |
48.3 |
67 |
57 |
7.8 |
RR |
100.2 |
128 |
116 |
14.3 |
||
Fall |
FF1 |
76.1 |
131.1 |
98 |
24.3 |
Spring |
FF1 |
68.4 |
173.4 |
116.7 |
41.1 |
FF2 |
74.6 |
127.3 |
96.9 |
23.6 |
FF2 |
67.9 |
165.7 |
114.6 |
38.5 |
||
VV |
44.1 |
69.2 |
57.9 |
12.6 |
VV |
58.7 |
104.9 |
82.3 |
22.1 |
||
RR |
48.2 |
66.3 |
57.1 |
9.2 |
RR |
64.3 |
109.8 |
80.5 |
20.9 |
Table 5 Results of the analysis of conductivity, unit µS.cm-1. Source: the author herself
PT, point; Min, minimum value; Max, maximum value; SD, standard deviation
The highest values recorded for conductivity correspond to the FF1 and FF2 points in Spring and Winter. The point of VV and RR showed the highest conductivity rate in Winter. The lowest rates recorded correspond to the VV points in the Fall and RR in the Summer and Fall. The FF1 and FF2 points behaved in a similar way throughout the seasons and showed a constant growth until the Winter. The behavior of the VV and RR points was also similar during the seasons of the year, and Winter had the highest average rates. The Summer and Fall had similar data for these same points, with a rise in conductivity in Winter and a subsequent reduction in the Spring.
The NBR 15.527/2019 and Consolidation Ordinance nº 888/2021 that were selected for this study, do not address the question of the electric conductivity parameter. The results for this parameter are usually lower than 100 µS.cm-1 in natural waters although water bodies that receive a high load of domestic and industrial wastewater, can reach 1000 µS.cm-1, which is a sign of a serious pollution problem (LIBÂNIO, 2010; HAGEMANN and GASTALDINI, 2016). The Summer and Fall showed averages below 100 µS.cm-1, for all the points and Spring had averages for the VV and RR points as well.
The highest average rates of electric conductivity were recorded in Winter and Spring. In the region under study, Winter had the lowest volume of total precipitation between 2017 and 2018, to such an extent that it can be characterized as a serious dry period and hence leads to an increase in the concentration of pollution. Marques et al.20 noted that there were higher rates of electric conductivity in periods of gentle rain or lack of rainfall and stated that the chemical composition of rainfall varies when there are consecutive days either without rain or experiencing a heavy downpour. The author added that there is a greater absorption of solar radiation by gases and small particles in the atmosphere during what is regarded as periods of gentle rainfall. The rainwater collected and analyzed in the study by Andrade et al.21 showed greater conductivity when there was a longer interval between the precipitation events.
Total Alkalinity
The alkalinity divided by seasons (Figure 7) for the FF1 point ranged from 22.3 to 81.5 mg. L-1, with an overall average of 40.30 ± 13.51 mg.L-1. At the FF2 point, the variation of the results was from 23.6 to 73.7 mg.L-1, with an average of 41.26 ± 13,97 mg.L-1, while the VV point recorded a minimum value of 16.7 mg.L-1 and maximum of 59.4 mg.L-1, with an average of 32.57 ± 12.12 mg.L-1. The data for the RR fluctuated between 5.4 and 49.9 mg.L-1, and the average was 30.03 ± 12.01 mg.L-1.

Figure 7 Boxplot of the alkalinity at the FF1, FF2, VV and RR collection points, corresponding to the seasons of the year. Source: the author herself.
The variations of the results obtained from the total alkilinity during the seasons of the year is shown for each collection point in Table 6.
SEASON |
PT |
DATA |
SEASON |
PT |
DATA |
||||||
Min. |
Max. |
Average |
SD |
Min. |
Max. |
Average |
SD |
||||
Summer |
FF1 |
22.32 |
52.38 |
35.52 |
9 |
Winter |
FF1 |
25.38 |
63.99 |
42.48 |
11.53 |
FF2 |
23.58 |
48.87 |
33.74 |
7.78 |
FF2 |
31.05 |
64.26 |
44.83 |
12.09 |
||
VV |
16.74 |
36.72 |
23.67 |
6.56 |
VV |
29.16 |
49.41 |
40.73 |
6.73 |
||
RR |
5,.0 |
38.34 |
22.2 |
10.92 |
RR |
41.31 |
49.68 |
44.37 |
4.62 |
||
Fall |
FF1 |
24.3 |
45.36 |
37.48 |
9.21 |
Spring |
FF1 |
29.97 |
81.54 |
51.98 |
24.85 |
FF2 |
25.92 |
46.71 |
38.61 |
9.96 |
FF2 |
30.24 |
73.71 |
53.95 |
20.98 |
||
VV |
17.28 |
31.86 |
25.49 |
6.44 |
VV |
23.22 |
59.4 |
42.34 |
14.99 |
||
RR |
16.2 |
30.78 |
24.99 |
6.61 |
RR |
25.38 |
49.95 |
37.33 |
10.23 |
Table 6 Results of the analysis of alkalinity, unit mg. L-1 of CaCO3. Source: the author herself
PT, point; Min, minimum value; Max, maximum value; SD, standard deviation
The widest dispersal of the results was found in Spring at the FF1, FF2 and VV points. The RR point showed the greatest dispersal in Summer. The highest values of alkalinity can be found in the Winter and Spring. In general, there is a drop in alkalinity from the VV and RR points following the passage from the FF1 and FF2 points in the Summer, Fall and Spring. It was noted that generally there is an increase in alkalinity during the seasons of the year starting from Summer and going on to Spring. The maximum values of alkalinity were higher at the FF1 and FF2 points in all the seasons, the highest recorded occurring in Spring. The lowest value was found at the RR point in Summer.
The NBR 15.527/2019 and Consolidation Ordinance nº 888/2021 selected for this study did not take account of the alkalinity parameter. In the case of most of the natural waters, the results were obtained in the range of 30 to 500 mg.L-1 of CaCO3. The value hardly ever exceeds 400 to 500 mg.L-1 of CaCO3 for water.22 The lowest value found in this study was 5.40 mg.L-1 of CaCO3 and the highest was 81.54 mg.L-1 of CaCO3. The averages ranged from 20 to 55 mg.L-1 of CaCO3.
The constituents responsible for total alkalinity, expressed in mg.L-1 of CaCO3, are biocarbonates (HCO3-), carbonates (CO3-2) and hydroxides (OH-). The distribution of alkalinity is known from the pH, and can be shown as follows: pH > 9.4 (hydroxides and carbonates), pH between 8.3 and 9.4 (carbonates and bicarbonates) and pH between 4.4 and 8.3 (only bicarbonates).22 On the basis of the pH results of this study, it was confirmed that the total alkalinity of the samples is, in practice, related to the bicarbonates (HCO3-). Most of the averages of this parameter were obtained in Winter and Spring, and followed a similar pattern to conductivity.
Alkalinity is directly correlated to pH, or in other words, the lower the value of the pH, the less is its capacity to neutralize an acid. The following are required to analyze the overall averages of the pH: an FF1 value of 7.26 ± 0.71, an FF2 equal to 7.37 ± 0.93, a VV value of de 7.28 ± 0.49 and an RR equal to 7.19 ± 0.44. When this is analyzed together with the averages obtained for alkanility, it is clear there is a decline in this parameter from the points of the first flush (FF1 and FF2) to the points of the tank (RR and VV). This suggests that there is a reduction in the capacity for neutralizing the stored water, depending on the occurrence of rainfall, with the exception of the season with the longest period of dry weather which is the Winter. It was clear that there was a similar pattern of behavior during the seasons from the FF1 to the FF2 point, as also between the VV and RR points.
Chloride
During the annual analysis of the seasons, the chloride (Figure 8) fluctuated at the FF1 point from 0.00 to 103.8 mg.L-1, with an average of 12.08 ± 19.86 mg.L-1. The results at the FF2 point ranged from 0.0 to 31.5 mg.L-1, with an average of 9.33 ± 7.80 mg.L-1, whereas the VV point had a minimum value of 0.00 mg.L-1 and a maximum of 22.42 mg.L-1, with an average of 5.58 ± 5.78 mg.L-1. The RR data fluctuated from 0.0 to 88.2 mg.L-1, with an average of 9.37 ± 20.26 mg.L-1.
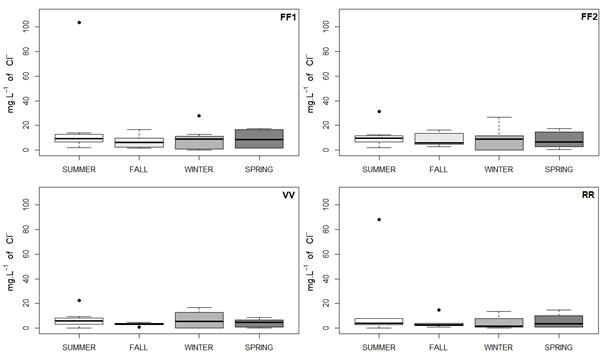
Figure 8 Boxplot of chloride from the collection points of FF1, FF2, VV and RR, depending on the seasons of the year. Source: the author herself.
Table 7 shows the minimum and maximum values, the average and the standard deviation corresponding to the seasons of the year.
SEASON |
PT |
DATA |
SEASON |
PT |
DATA |
||||||
Min. |
Max. |
Average |
SD |
Min. |
Max. |
Average |
SD |
||||
Summer |
FF1 |
1.95 |
103.76 |
18.92 |
32.06 |
Winter |
FF1 |
0 |
27.88 |
8.72 |
9.88 |
FF2 |
1.95 |
31.5 |
10.94 |
8.35 |
FF2 |
0 |
26.61 |
8.44 |
9.71 |
||
VV |
0 |
22.42 |
7.23 |
7.36 |
VV |
0 |
16.86 |
6.77 |
7.47 |
||
RR |
0 |
88.2 |
17.82 |
34.57 |
RR |
0 |
13.65 |
5.1 |
7.45 |
||
Fall |
FF1 |
1.46 |
16.87 |
7.29 |
6.3 |
Spring |
FF1 |
1.66 |
17.55 |
9.24 |
7.71 |
FF2 |
2.92 |
16.18 |
8.64 |
5.89 |
FF2 |
0.29 |
17.55 |
8.38 |
7.48 |
||
VV |
0.97 |
4.58 |
3.12 |
1.36 |
VV |
0 |
8.77 |
4.29 |
3.75 |
||
RR |
0.97 |
14.62 |
4.87 |
5.56 |
RR |
0.68 |
14.62 |
5.53 |
6.51 |
Table 7 Results of the analysis of chloride, unit mg. L-1 of Cl-. Source: the author herself
PT, point; Min, minimum value; Max, maximum value; SD, standard deviation.
Most of the averages in all the points were recorded in Summer, in particular in the case of the FF1 and RR points. In general, there was a reduction in the concentration of chloride at the FF1 and FF2 points compared with the VV and RR points. Some of the results had a standard deviation that was above average, which suggests there was a wide variation of data during the analyses. In the case of the FF1 and FF2 points, there was a reduction of chloride from the Summer to the period of the Fall but it remained stable during the Winter and Spring. There was a reduction of chloride in the Fall with regard to the level of the Summer and subsequently, there was a rise in the Winter at the VV and RR points, with a reduction of these ions in Spring at the VV point, while the average was kept at a stable level at the RR point in the Spring.
The Consolidation Ordinance nº 888/2021 fixes the rate at 250 mg.L-1 of Cl-. All the results are within the limits set for the seasons of the year and the highest value found was 103.76 mg.L-1 of Cl-. The values found can be regarded as low when compared with sea water where there is a greater concentration of chloride – around 26,000 mg.L-1. Its use might be restricted when it shows high results of chloride owing to the addition of taste to the water and its laxative effect (BRASIL, 2013). Systems installed near to oceans tend to have features that are in accordance with the concentrations that are found in sea water.23 Thus it was inferred that the presence of chloride in the samples that were analyzed, is the result of their geographical location since the city of Rio de Janeiro is close to the Atlantic ocean. In addition, chlorides can be found in the excrement of animals which causes the pollution of the water (BRASIL, 2006).
A correlation matrix using parameters of the seasons of the year
The purpose of carrying out a correlation matrix study is to determine a linear relationship between variables. The degree of relationship is quantified by the correlation coefficient (r), which describes the link between the two variables. The values of this coefficient can range from -1 (or -100) to +1 (or +100), and can be directly or inversely proportional. Their proximity to these limits indicates a greater linear relationship while their proximity to zero indicates the lack of an association.24
The analyses of the relationships between variables was carried out by means of the correlation martrix and employing the Pearson method, in a centesimal scale ranging from -100 to 100. The graphic representation consists of ellipses, variation of colors and the correlation coefficient (Figure 9). The ellipses show the magnitude and direction of the relationship. The more flattened the ellipse is, the stronger the correlation, since the correlation is regarded as being perfect when it is represented by the gradient of a straight line. With regard to the direction, the lines are directly proportional to those that show a rising slope (“/”) and inversely proportional to those that show a descending slope (“\”). The color assists by providing a purposeful visualization in which a tendency to red indicates the greater intensity of a direct relationship, while the tendency to blue shows a greater inverse association. A perfect direct correlation is established when the coefficient is equal to 100, while a relationship is regarded as inversely proportional in a perfect way if it is equal to -100.25
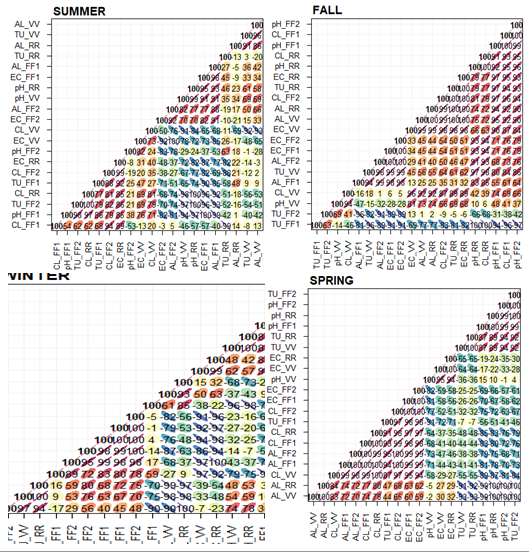
Figure 9 Correlation matrix shown for the season of the year and containing the Pearson´s coefficient (r). Source: the author herself.
For the purposes of analysis, correlations were selected that have a powerful magnitude with regard to the seasons of the year, with an emphasis being laid on the r > 90 coefficient. A powerful direct correlation indicates that the high (or low) values of a variable are related to the high (or low) values of another variable. However, an inverse association indicates that the high (or low) values of a variable are associated with the low (or high) values of another one.
It was found that in the Summer there was a strong direct correlation between conductivity and alkalinity at the FF1 point (r= 98) and the FF2 point (r= 92), which suggests that the bicarbonates (HCO3-) had an influence on the samples that were analyzed. There was a highly positive linear relationship between the pH at the VV point and the pH at the RR point (r= 99), which suggests there was a similar pattern of behavior in these places. Strong inverse associations were found in the turbidity and conductivity pairing at the FF1 (r= -90), which suggests there was greater turbidity and less conductivity at this point. An intense inverse relationship was also found in a) the pH and conductivity pairing at the FF1 point (r= -100), b) the conductivity from the FF2 point and c) conductivity from the VV point (r= -92), d) alkalinity and chloride from the VV point (r= -93) and e) the pH and chloride pairing at the VV point (r= -91).
With regard to the Fall, there was a close direct correlation between: a) the pH and chloride pairing at the FF1 points (r= 100), VV (r= 94) and RR (r=100), b) the pH of FF1 with pH of FF2 (r= 99), c) conductivity and alkalinity from the FF1 (r= 98), FF2 (r= 100), VV (r= 99) and RR points (r=100). Other pairs showed a highly positive correlation, such as the following: conductivity from FF1 and conductivity from FF2 (r= 100), alkalinity from FF1 with the alkilinity from FF2 (r= 99), chloride FF1 and chloride FF2 (r= 96), conductivity and chloride from the VV point (r= 96), alkalinity from the VV with alkilinity from the RR (r=100), turbidity with conductivity of the RR (r= 100) and with alkalinity (r= 100). Strong inverse associations were observed at the FF1 in the pH and turbidity pairing (r= -94), turbidity and conductivity (r= -91) and conductivity and chloride (r= -96). Highly negative correlations were found between the turbidity of FF1 and the turbidity of VV (r= -96), and both the turbidity and alkalinity of the FF2 point (r= -91).
In the case of Winter, there was a highly positive magnitude between the pairs of conductivity and alkalinity at the FF1 points (r= 100) and FF2 (r= 100), as well as the following: conductivity from FF1 and conductivity from FF2 (r= 98), allkalinity from FF1 and alkalinity from FF2 (r= 100), chloride from FF1 with chloride from FF2 (r= 99), chloride from VV (r= 99) and chloride from RR (r= 100). As well as these, the FF2 point showed a close correlation between the turbidity variable and conductivity (r= 95) and with alkalinity (r= 98). There was a very close relationship between the FF2 chloride parameter and the chloride from the VV (r= 100) and RR (r= 99), as well as between the pH and the chloride at the VV point (r= 94) and pH of the VV with the pH of the RR (r= 100). There were negative associations between the pH and chloride at the FF1 point (r= -98), conductivity from the FF1 with the conductivity from the VV (r= -94) and RR (r= -98), as well as between the conductivity from the FF2 and conductivity from the RR (r= -94) and turbidity and alkalinity from the RR point (r= -97).
Spring showed strong positive realtionships at the FF1 point of the turbidity variable with conductivity (r= 98), alkalinity (r= 94) and chloride (r= 93), as well as conductivity with the alkalinity of FF1 (r= 99), chloride of FF1 (r= 98), conductivity of FF2 (r= 100), alkalinity of FF2 (r= 99) and chloride of FF2 (r= 100). Other variables also had an intensely positive correlation, such as the pH between the FF1 and FF2 points (r= 99), chloride of the FF1 point with chloride of FF2 (r=99), VV (r= 99) and RR (r= 100). It was confirmed that there was a high direct correlation between the pH variable and the turbidity of the FF2 point (r= 100), and VV point (r= 94) and the pH of the RR point (r= 99), as well as the turbidity of the FF2 point with the turbidity of VV (r= 92) and RR (r= 92). High direct correlations were also obtained by the conductivity with the alkalinity of the FF2 point (r= 99) and with the chloride of FF2 (r= 100), chloride of the FF2 point with the chloride of the VV point (r= 97) and RR point (r= 98). It should also be noted that there was a high positive realtionship between the pH and the conductivity of the VV point (r= 95), turbidity of the VV point with the turbidity of the RR point (r= 100), conductivity between the VV and RR points (r= 100), alkalinity between the VV points and RR points (r= 100) and chloride between the VV and RR points (r= 100). Turbidity with alkalinity from the VV point (r= -93), were selected for the inverse associations, together with pH with alkalinity from the RR point (r= -100) and turbidity with alkalinity from the RR point (r= -92).
In generic terms, it was possible to determine the influence of the ions on the correlations of the data, where the conductivities were positively related with the alkilinity and chloride. In some of the correlations drawn on for discussion, there proved to be a direct correlation between the FF1 and FF2 points, as there was between the VV and RR points, and an inverse relationship between the points of first flush (FF1 or FF2) with VV or RR. The highest degree of correlation was found in the Fall and the lowest in Spring, which suggests a greater correlation. It was also confirmed that there were values close to zero in the graph which suggest a wider dispersion of the results and a low correlation. In general, it can be concluded that there is a greater variability in the way the correlation of the parameters is distributed in the Fall and Spring since these are transitional seasons, while in Summer and Winter there is a relatively more uniform pattern of behavior since they are characterized by periods of more prolonged periods of rainfall or dry weather, respectively.
The principal components analysis (PCA)
The principal components analysis (PCA) was conducted with the aim of carrying out a multivariate modelling and a better visualization of the data. This statistical method seeks to employ decorrelation techniques and reduce the number of original variables by creating new variables (known as principal components), which are able to explain how the maximum variablity of data can be obtained with the lowest possible loss.26 The 1-2 components were used in this study because they were regarded as of sufficient scope to provide the highest percentage of results. They were represented in a two-dimensional graph (Figure 10), in which the first principal component (PC1) is displayed in a horizontal axis, and is responsible for a wider variation of data. The second component (PC2) is orthogonal to the first, and appears in the vertical axis where it shows the second largest variation in the data, since it is a residual variation that cannot be determined in advance by the PC1.26
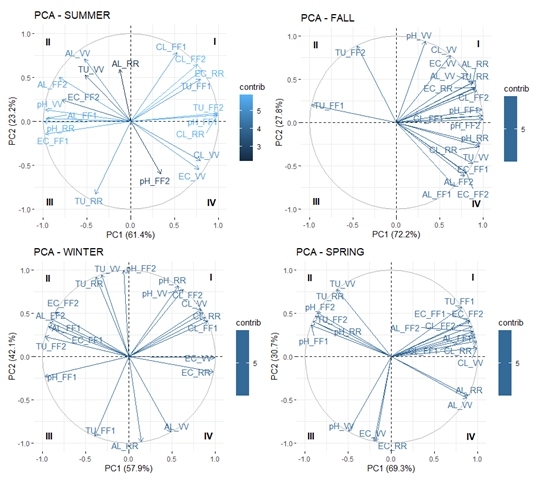
Figure 10 Distribution of the variables for the water quality for the seasons of the year, based on the PCA method. Source: the author herself.
The results were based on 13 days of collection over a period of two years and obtained from 4 points, making a total of 52 samples for an analysis of principal components. The pattern for the number of collections for the seasons of the year was as follows: 4 for Summer, 3 for the Fall, 3 for Winter and 3 for Spring. During the Summer, there was found to be a degree of alkalinity in Quadrant II, as well as chloride and conductivity in Quadrants I and IV at the VV and RR points. The pH, turbidity and chloride variables led to the formation of the positive axis of PC1 while the pH, conductivity and alkalinity are related to the negative axis. In the case of PC2, it was found that the chloride and alkalintiy variables were in the positive axis and turbidity, pH and conductivity in the negative axis.
In the Fall, there was a close association between the electric conductivity and alkalinity variables located in Quadrants I and IV, in all the points. The pH and chloride parameters were able to form a PC1 axis, while the turbidity was responsible for a negative axis. The PC2 was defined in a positive way by the pH, turbidity and chloride variables and negatively by the alkalinity and conductivity.
There were strong correlations in Winter between: the pH of the RR and VV points and chloride in all the points in Quadrant I. In Quadrant II there was a close association between the turbidity of the VV and RR points and between the alkalinity and conductivity of the FF1 and FF2 points. Conductivity and chloride were the variables that led to the positive axis of the PC1, particularly in the case of the VV and RR points, whereas pH, turbidity, conductivity and alkalinity led to the negative axis, with the assistance of the first flush points. The main contributory factor to the positive axis of the PC2 was found in the pH and turbidity parameters, and alkalinity and turbidity were responsible for the negative axis.
In Spring, there was a notably close relationship between the following: a) conductivity, alkalinity and chloride in Quadrant I for the FF1 and FF2 points, b) pH and turbidity in Quadrant II for the FF2 and RR points, c) conductivity and pH in Quadrant III for the VV point, and d) alkalinity in Quadrant IV for VV and RR. The chloride and alkalinity variables were the main factors responsible for the positive axis and the pH for the negative axis of the PC1. The turbidity variable was a key factor in the formation of the positive axis of PC2, and conductivity and alkalinity for the negative axis.
In general, it was determined that a contributory factor made by the principal components was that they led to a greater interference of the dissolved ions that were present in the rainwater which corresponded to the variables that were mainly responsible for the formation of the principal axes. However, it could also be seen that there was interference by the suspended solids in the PC2 during Winter and Spring.
It should be noted that the variables with a vector of a greater length, are those that are more likely to lead to the formation of the principal components. Thus, the variables that can be found nearest the unit circle and the axes of the components, are more representative. In addition, the variables located nearest to each other have a greater correlation and the overlapping of the variables is a sign of a similarity with regard to their graphic representativeness.27 The positive values for the PC1 can be found in Quadrants I and IV, while in the case of PC2, they are located in Quadrants I and II. Quadrant I is positively correlated with PC1 and PC2 and Quadrant III is negatively correlated with both. This technique also allows a set of variables to be formed on the basis of their associations. In general, a set of representative variables of a more intense kind were found in the Fall and Spring and this explains why there was a lower dispersal of air pollutants during the hydrological year.28–40
It can be concluded from this study that after the results obtained over a period of two years had been analyzed, generally speaking:
the water stored in the tank was of a satisfactory quality for mundane purposes;
there was a reduction in turbidity and the RR point conformed to the maximum value laid down by the adopted standards and on the whole, there was a reduction of conductivity;
It is recommended that microbiological analyses should be conducted throughout the seasons of the year in a way that can serve as a basic parameter in the use of water for more serious purposes such as for the irrigation of fruit plants. Apart from this, there is a need to learn how to use water only for non-drinkable purposes owing to the risk of contamination from rainwater caused by impurities on the surface of the catchment area and atmospheric pollution. It is also suggested that the study could be supplemented with further physico-chemical parameters.
We would like to express our thanks to FAPERJ - Fundação Carlos Chagas Filho de Amparo à Pesquisa [Carlos Chagas Foundation for the Support of Research] in the State of Rio de Janeiro for providing grants from the Technology Initiation Project 2018/2, Process No. 260.025/2018, FAPERJ - ADT1-2015/2 and Mobile Digital Technologies for water safety and the system for the catchment and storage of rainwater, Process 010.001985/2016 for their financial support which enabled us to undertake this study.
We are also grateful to i) CNPq [National Council for Scientific and Technological Development], ii) Chamada Universal MCTI/CNPq on 14/2014 with Process No. 457688/2014-9 iii) FINEP [Funding Agency for Research Studies and Projects]; iv) the Projeto de Manejo de Águas Pluviais em Meio Urbano (MAPLU) [Project for Handling Rainwater in the Urban Environment], and v) Chama Pública de Saneamento Ambiental e Habilitação [Public Call for a Sanitary Environment and Housing] on 07/2009. The authors are also grateful to the technicians at the Laboratory of Sanitary Engineering (LES), at the Engineering Faculty of the State University of Rio de Janeiro, for their ex situ laboratory analyses of the parameters discussed in this article.
The author declares there is no conflict of interest.

©2022 de, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.