International Journal of
eISSN: 2576-4454


Research Article Volume 3 Issue 5
1Department of Comparative Biology, Faculty of Sciences, National Autonomous University of Mexico, Mexico
2Hydrobiology Laboratory, Institute of Biology, National Autonomous University of Mexico, Mexico
Correspondence: María Edith Ponce Marquez, Department of Comparative Biology, Faculty of Sciences, National Autonomous University of Mexico (UNAM), Mexico, Tel +52 55 56 22 54 01
Received: August 13, 2019 | Published: September 11, 2019
Citation: Marquez MEP, Espino MDLL. Phytopanktonic composition and indicative species of water quality in high artificial ponds at Mexico city. Int J Hydro. 2019;3(5):345-349. DOI: 10.15406/ijh.2019.03.00198
This study analyses the water quality and microalgae composition of an artificial reservoir in the Mexican basin called La Cantera, built in 2005. Thirteen years later (2018) it shows a change in both its chemistry and microalgae structure and a visible deterioration (eutrophication) due to several nearby internal academic and anthropogenic activities. This study has therefore assessed water quality by measuring concentrations of nitrogenated and phosphorus total inorganic nutrients (>500µM and 120µM, respectively) that showed an evident eutrophication. During our seven-year sampling period we found the following composition and predominance of algae of the taxa: Bacillariophyceae, Cyanoprokaryota and Chlorophyta. Results showed a predominance of the first biological taxa with nine indicative species of different contamination types with a preponderance of Navicula, and two in the case of the second taxa with a predominance of Microscystis.
Keywords: La Cantera, artificial reservoir, eutrophication, bacillariophyceae
Epicontinental bodies of water can be natural (e.g. lakes) or artificial (man-made). Studies on phytoplanktonic composition carried out in these bodies of water have been fundamentally taxonomic, and only until the beginning of this century they have focused on ecologically characterizing aquatic environments and using some genera and, specially, on pollution-indicator species. It should be noted that much of the composition is a consequence of several aquatic environmental factors such as physiochemical and climatic characteristics which adequate to phytoplanktonic blooms, mortality or natural time-space succession of phytoplankton. In man-made reservoirs, the variety of anthropogenic activities has changed these characteristics exceeding the recuperation capabilities or resilience of the chemical and biological composition.
Some artificial reservoirs or dams were built many centuries ago as water reservoirs for humans and their economic activities, as flood control, agricultural irrigation, electricity generation, sports and tourism, among others, activities that have generated all kinds pollution. A recent artificial reservoir is the A3 Buffer Zone inside the Pedregal de San Angel Ecological Reserve where the Cantera Oriente is located. The Cantera was given in concession to the federal government by the National Autonomous University of Mexico (UNAM) for the extraction of volcanic rock to produce asphalt used in construction.1 This industry was later abandoned and an aquatic system with exotic vegetation was created for research, teaching, conservation and recreation purposes. This author (Lot) had said that the Cantera, due to its origin, was a very damaged site and a real challenge for any lake landscape creation program; nevertheless, its ecological rehabilitation was attempted.
Given the interest in understanding the environmental dynamics of La Cantera, a number of individual studies have been developed in several specialties such as: geology, physics, chemistry and biology. However, most of these studies have been isolated, descriptive and, unfortunately, without integration. For example, specific biological studies on phytoplanktonic composition have been conducted in short periods of time, and in some of these, abundance has been associated with eutrophication. Water quality or physiochemistry has frequently been considered exclusively within the description of the study area and, in the best of cases, it has been used to briefly explain the presence of some species. The aim of this paper is to analyze the physiochemistry of the water and define the phytoplankton composition to gender level, and to find the indicator species of water quality.
Study area
La Cantera was originally a place operated by an asphalt plant until a huge 16hectares and 42meters deep hole was created. After exploiting it for 25years and seeing that water was beginning to leak from the bottom, it was finally closed. In 1996 the property was returned to the National Autonomous University of Mexico, UNAM (personal communication from Biologist Francisco Martínez, in charge of the place http://www.unamglobal.unam.mx/?p=46649). Today it is an artificial reservoir located southwest of Mexico City (19° 17' N, 99° 11' W) within the University campus. It consists of five bodies of water surrounded by a 40m high basalt wall, a total area of 11,906.45m2 and an average depth of 2m1 with large photoplanktonic growths. The surrounding vegetation is composed of xerophytic tree scrub with cypresses, tulars (herbaceous plants rooted in the bottom of swampy land or on the banks of lakes), as well as reforestation areas (Figure 1). On the surrounding area there is a dense urban population and a wide street with heavy vehicular traffic. The climate is temperate sub-humid with summer rains [Cb(w1)w], and heavy rainfall from June to October and a dry season from November to May. Average annual temperature is 15.6°C and an average annual rainfall of 833mm. It consists of volcanic rock substrate originated from the eruption of the Xitle and Ajusco volcanoes about 1670years ago. Soil is scarce and shallow (Descriptive sheet of the Ecological Reserve of Pedregal de San Angel REPSA consulted in March 2019 http://www.repsa.unam.mx/index.php/ubicacion/mapa-del-sitio/44-2013-08-07-13-15-59/29).There is a seasonal loss of the water mirror. This artificial body is fed by springs and cracks from the Ajusco Hill.
Water samples were collected for the phycological study and physiochemical characteristics of water quality for seven years (2006, 2010, 2011, 2012, 2013, 2014, 2016), to estimate changing tendencies and, as far as possible, the distribution and association with physicochemical properties to be used as indicators of eutrophication, in 10 sites chosen for their geomorphology: five in lotic areas (runoff zones and natural and artificial canals) and in five lentic environments (Figure 2).
Field work
Temperature, pH, conductivity and dissolved oxygen measurements were taken in situ with a conductometer/potentiometer (Hach). Chemical analyses were done on water samples showing the presence of nitrates, nitrites, total phosphorus, hardness, silicates, among others (with the Hach portable equipment). The visible epiphyte algae material was collected by hand with spatula and tweezers from a 4cm2 area. Diatoms were collected from rock scrapings. Perifiton (epiphytic algae) was obtained by compressing some of the surrounding vegetation. On water from the lake we used a phytoplankton net (with a mesh opening of 10microns) which could be dragged approximately three meters.2 Samples were kept in 30ml/50ml polyethylene bottles with 3% formalin. For diatom determination (essential component of the algal community), organic matter was eliminated using the acid oxidation technique; mounted on synthetic resin.3
The Hoek et al.5 scheme was used to identify the genera besides the following fundamental references for Cyanoprokaryota: Komárek et al.,5 for Bacillariophyceae. Round et al.,6 for Xanthophyceae, Ettl,7 for Chlorophyta, Ettl and Gardner ,8 among others.
Variations in conservative parameters such as temperature and conductivity showed a wide variation between 12 to 20°C and 400 to 528µS/cm2 respectively as a result of seasonal and interannual seasonality, evident even during the time of measurement taking. Conductivity levels exceeded those established for freshwater (100µS/cm2); however, total hardness ranged from 68 to 170mg/L so the water from the La Cantera can be considered from soft, semi-hard to moderately hard. Silicate concentrations ranged from 18 to 45mg/L, the latter being due to the high density of diatoms. Concentrations of nitrogenated nutrients (nitrites, nitrates, ammonium) and total phosphorus were variable and generally high, exceeding the contents of natural water, making the Cantera a hyper-eutrophicated place (Table 1).
Temperature °C |
20-Dec |
Conductivity µS cm-1 |
400-528 |
SiO4 mg/L |
13-61 |
pH |
6.0-10 |
Nitrates µM |
Oct-57 |
Nitrites µM |
0.2-8.0 |
Total Phosphorous µM |
35-142 |
Ammonioum µM |
6.0-27.0 |
Silicates mg/L |
8.4-62 |
Hardness mg/L |
68-170 |
Table 1 Physiochemical characteristics of the Cantera pond water
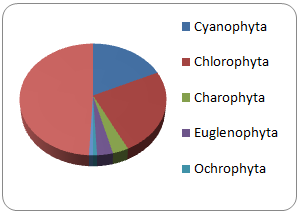
The largest taxa from the six identified in the La Cantera in 2006 and 2016 was Bacillariophyceae (49 and 81%, respectively), followed by Cyanoprokarota (18 and 14%), Chlorophyta (25 and 50%). The rest were: Euglenophyta, Ochrophyta, Xantophyceae the less abundant (Figure 3). An analysis of all sampled years, within the Cyanoprokarota, the predominant genera were Anabaena, Phormidium, Chroococcus and Microscystis, followed by Planktothrix and Scytonema. The important genera of Bacillariophyceae were Achnanthes, Aulacoseira, Cyclotella and Rhoicosphenia. Of Euglenophyta: Euglena and Phacus with low percentages. The Ochrophyta Division and the genera Xenococcus from Cyanoprokarota were the least abundant.
There is no comparative resemblance to other natural or artificial bodies of water. The composition of microalgae genera in La Cantera artificial reservoir is evident, and it can be the result of several environmental factors inside and out this body of water. In addition, space-time variability of both abiotic and biotic factors and the intrinsic conditions of the genera and their life cycles also play a role. Oliva Martinez et al.,9 generalizes to the level of country stating that phytoplankton is mainly composed of the taxa Bacillariophyceae (31.9%), Cyanophyceae (18.24%) and Chlorophyceae (17.4%), within which there are genera or species indicative of water quality. Valeriano Riveros10 classifies the percentage of the number of genera in the lake at Valle de Bravo as follows: Cyanophyceae 26%, Chlorophyceae 34.33%, Bacillariophyceae 13.12%, Euglenophyceae 3.3%, Xantophyceae 1.1%, other taxa complete 100%. The phytoplanktonic composition in La Cantera is shown in Figure 3.
|
2006 |
% |
Cyanophyta |
22 |
18.03 |
Chlorophyta |
30 |
24.75 |
Charophyta |
4 |
3.27 |
Euglenophyta |
4 |
3.27 |
Ochrophyta |
1 |
0.83 |
Cryptophyta |
0 |
0 |
Xantophyceae |
1 |
0.83 |
Bacillariophyceae |
60 |
49.02 |
|
122 |
100 |
|
2016 |
% |
Cyanophyta |
14 |
8.00 |
Chlorophyta |
50 |
32.84 |
Charophyta |
5 |
3.13 |
Euglenophyta |
5 |
3.18 |
Ochrophyta |
0 |
0 |
Cryptophyta |
1 |
0.63 |
Xantophyceae |
1 |
0.63 |
Bacillariophyceae |
81 |
51.59 |
|
157 |
100 |

Figure 3 Comparative percentual composition and their pay representative distribution, between 2006 and 2016 of taxa found in La Cantera.
Several genera of the Bacillariophyceae were abundant during the seven-year sampling period: Ulnaria (4.59%), considered to belong to scarcely polluted environments (oligosaprobiont to beta-mesosaprobiont);11 Achnanthes (4.33%) is reported from less contaminated media but has been seen in places with scarce to heavy pollution;12 Aulacoseira (4.19%), its two species have been found in Lake Cocibolca, Nicaragua, polluted with organic residues (Hernández González and Guerrero Avilés repositorio.unan.edu.ni/2549/1/354.pdf);13 Rhoicosphenia (4.13%), a genus considered to belong to scarcely disturbed and well oxygenated water;14 Surirella (3.79%) important because they are usually found in lakes or bodies of water with high concentrations of salts or in shallow water;15 Nitzschia (3.90%) some of these species tolerate a wide range of environmental conditions16 and show hypertrophic conditions such as those registered at the Lerma River;14 Pinnularia (4.25%) can be considered optional or facultative heterotrophic diatoms which enables them to reproduce in extreme ecosystems;17 Sellaphora (3.79%), its species S. pupula is an indicator of water highly contaminated with industrial discharges (Segura García et al., 2012) and even domestic discharges, and reported by several authors as belonging to eutrophic and hypertrophic environments.18 Also, Cyclotella and Cymbella, in smaller percentages (between 3.67 and 3.80%). In La Cantera, Navicula had three species considered as indicators of poor water quality or pollution, along with Nitzchia and two species of Microscystis (Table 2).
Cyanoprokaryota |
Bacillariophyceae |
Microscystis novacekki |
Achnanthes exigua |
Microscystis protocystis |
Achnanthidium minutissimum |
Gomphonema gracile |
|
Gomphonema parvulum |
|
Navicula cryptotenella |
|
Navicula radiosa |
|
Navicula trivialis |
|
Nitzschia communis |
|
|
Nitzschia palea |
Table 2 Indicator species of water quality in La Cantera
Nitzschia palea is abundant in several sites highly contaminated by domestic wastewater. Many authors have written about it being typical of environments rich in organic matter.19‒21 Navicula mutica is also abundant in places with high conductivity and high nutrient concentrations.9 The genus Achnanthes, especially A. minuttissima, is common in a wide range of environmental conditions.Gomphonema gracile was the dominant species, typical of this place. Different authors22‒24 recorded this species in eutrophic lakes; they tolerate pollution and eutrophication, caused by nitrogen compounds, better.25
According to Duque and Donato,23 the species Coccinodiscus perforatus, Gomphonema gracile, G. parvalum, G. pseudoaugur and G. angustum, were only found in the eutrophic lake; these are found in water with high contents of organic matter from the discharge of effluents with faeces and blood from pig slaughter-houses. These species can quickly convert large quantities of phosphorus into polyphosphates.24,26 Stenoic species, indicators of eutrophication, were Coccinodiscus perforatus, Gomphonema gracile, G. parvalum, G. pseudoaugur, G. angustum.
In regards to cyanophytes, Peinador27 states that it is not possible to compare different sampling sites with the lists found in literature and that define the species and associations most frequently recorded in different areas because these lists are incomplete as, just as those found in this study, they belong to specific locations and are far from universal. This author also states that a species can be found in different contamination conditions that depend on other characteristics of the site or may even be different varieties of the same species. This could be quite possible given the taxonomic ignorance regarding this group of organisms in the tropics. In general terms, however, most of the genera of cyanobacteria recorded in this study have been reported by one or more authors as being typical of water contaminated with organic matter, for example Microcystis novasekki or M. protocystis.
Dere et al.16 wrote that some species from different Divisions, such as the genus Nitzchia, tolerate a wide range of hypertrophic conditions like those of the Lerma River Mexico,12 in this basin, small-sized species like N. palea and Eolimna, and medium to larger-sized ones like Ulnaria, and Nitzchia and Sellaphora, the latter from relatively less contaminated places, have been found. All of this underlines the coincidence of Nitzchia and Navicula being indicators in La Cantera of a polluted, nitrogen enriched environment.
Although several authors propose genera or species as pollution indicators, like Nitzchia or Navicula, some of them do not specify the type of contaminant which means there is not always a coincidence in the results as their presence or abundance depends on the integration of various environmental factors. In La Cantera there was a predominance of other genera of Bacillariophyceae (from the most abundant to the less abundant): Ulnaria, Achnanthes, Aulacoseira, Rhoicosphenia, Surirella, Pinnularia, Sellaphora and Nitzschia, the least frequent being Navicula. These genera were not associated to any particular nutrient, whether nitrogenous or phosphorous, as these nutrients far exceed the recorded concentrations (total nitrogen >500µM and total phosphorus maximums of 120µM), besides high silicate content (between 20 to 40mg/L), which did not limit the abundance of Bacillariophyceae and which originates in igneous rock with high silicate content. There is a predominance of diatoms with sufficient silicate contents to justify their high percentage.
The most prominent genera of the Cyanoprokaryota taxa were: Anabaena, (11.41%), Chroccocus (10.27%) and Microscystis (8.75%); these, according to Tomasini Ortiz and Pérez Morales et al.,28 produce toxins that affect not only the zooplankton29 but also humans. Although they were found in high percentages, they still do not pose a danger to the zooplantonic community in La Cantera. It should be said that high concentrations of ammonium-ion were recorded in La Cantera; this ion inhibits the growth of cyanophytes, as recorded by Vintila and El-Shehawy (2007) with the species Nodularia spumigena, which is cosmopolite.
The chlorophytes that stood out in La Cantera were: Cladophora and Rhizoclonium, filamentous algae very abundant due to high concentrations of nitrogenated and phosphorated nutrients, as was seen on the rocky wall with percolations and runoffs, possibly fracture from the Ajusco hill. In this sampling site, during certain periods of time, a low dissolved oxygen content (30% saturation) was recorded, probably due to the input of organic matter used by this gas during its degradation.30,31
The most frequent and abundant taxa was Bacillariophyceae with nine indicator species, the two most important ones of Navicula and Nitzschia, followed by the taxa Cyanoprokaryota with only two species, mainly of Microscystis which determined the poor water quality and which are indicators of eutrophication from nutrients (nitrogen and phosphorus) and physical factors (conductivity, temperature), from the runoffs of peripheral urban settlements and their activities, and from springs possibly from infiltrations and cracks in the Ajusco hill whose skirts have begun to be urbanized. The genera Anabaena and Chroccocus can also pollute the water.
The authors would like to thank Angel Ceballos and Salvador Hernández Pulido for their help with the tables and figures.
The authors declare there is no conflicts of interest.
None.

©2019 Marquez, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.