International Journal of
eISSN: 2576-4454


Short Communication Volume 2 Issue 4
1Community Ecology and Conservation Group, Center for Integrative Conservation, Xishuangbanna Tropical Botanical Garden, Chinese Academy of Sciences, China
2Ecosystems Analysis Laboratory, Department of Botany, Banaras Hindu University, India
3Natural Resource Management Laboratory, Department of Botany, University of Delhi, India
4Institute of Environment and Sustainable Development, Banaras Hindu University, India
Correspondence: Chaturvedi RK, Community Ecology and Conservation Group, Center for Integrative Conservation, Xishuangbanna Tropical Botanical Garden, Chinese Academy of Sciences, China, Tel +86 18288059250
Received: August 04, 2018 | Published: August 29, 2018
Citation: Chaturvedi RK, Pandey SK, Bhadouria R, et al. Phenotypic plasticity of morphological traits determine the performance of woody species in tropical dry forest. Int J Hydro. 2018;2(4):516-518. DOI: 10.15406/ijh.2018.02.00119
In the extremely variable and severe environment of tropical dry forest, the phenotypic plasticity of morphological traits of plant species plays an important role for their development, functioning and evolution. We selected eight morphological traits (viz, girth, GT; height, HT; bark thickness, BT; wood specific gravity, WSG; leaf area, LA; crown cover, CC; crown depth, CD; leaf area index, LAI), which are considered important for the survival and growth of plant species in tropical dry forest, and measured their range in woody species, including trees and shrubs, across the five study sites. We also, measured phenotypic plasticity of morphological trait of each woody species across the study sites. In our study, the woody species exhibited wide range of morphological traits. The phenotypic plasticity of the morphological traits showed remarkable variation across species as well as within a particular species growing at different at different levels of soil moisture content. The relationships of mean value of morphological traits across all woody species at five study sites with the respective mean value of soil properties were significant. Among the selected traits, LA and HT were most important for the variation in SR at our study sites. We suggest extensive investigation of the phenotypic plasticity of the morphological traits of woody species in tropical dry forest for proper management and sustainable development of the forest ecosystem.
Keywords: phenotypic plasticity, morphological traits, tropical dry forest, soil moisture content
Although plant species share the key functional purpose, i.e., assimilation of photosynthetic carbon and transpiration, they exhibit wide variations in morphological traits.1-7 This property of a plant species to exhibit phenotypic variations according to changes in environmental conditions is commonly known as phenotypic plasticity of that plant species.8 The plasticity of any particular trait, possessing a genetic basis may also be adaptive, can increase the intensity or attenuate the evolved responses, and could itself show evolution in response to the selection on the basis of the range of spatial or temporal heterogeneity.7 The selection of functional traits can act on both forms, i.e., variation for traits and the variation for phenotypic plasticity of traits, therefore for understanding the evolution of plastic traits, it is necessary to investigate the complex interplay occurring between the plasticity in individual responses and the plasticity in evolved responses of populations. Interspecific correlations observed among the ecologically significant plant traits attract the attention of ecologists working on the evolutionary aspects because they may exhibit two distinct phenomena. First, they may indicate morphological, physiological or developmental ‘constraints’ which limit the independent variation as well as evolution of the associated traits for a particular environmental condition, and second, the correlations might be the adaptive outcome due to natural selection which may favour particular combinations of traits compared to others, and in such case the set of traits are commonly said to form an ecological ‘strategy’ dimension.9 Proper understanding of differences between these ecological explanations and analysing the factors affecting trait-based strategy dimensions is necessary because it provides us insight into life-history trade-offs which operate within as well as between environments, and also into important ecological phenomena for example, niche differentiation, species coexistence and the large shifts in plant traits which occur along geographic gradients. The study described in this communication was executed in the Vindhyan Highlands situated in Sonebhadra District of Uttar Pradesh, India (21º 29′–25º 11′ N and 78º 15′–84º 15′ E). For detail information about the various aspects of the study region, see Chaturvedi et al.10-16 On the basis of literature survey on tropical dry forests, we selected eight morphological traits (viz, girth, GT; height, HT; bark thickness, BT; wood specific gravity, WSG; leaf area, LA; crown cover, CC; crown depth, CD; leaf area index, LAI), which are considered important for the survival and growth of plant species in tropical dry forest, and measured their range in woody species, including trees and shrubs, across the five study sites. Further, we analysed the response of functional traits to variations in soil moisture content (SMC) across species as well as across study sites. For detail description of study design and the protocol for functional trait measurements, see Chaturvedi1 and Chaturvedi & Raghubanshi,17 Phenotypic plasticity of plant traits for each species across the study sites were calculated following Callahan18 as:
Results of the study showed that for trees, maximum GT (cm), HT (17m), BT (1.8cm) and LAI (14) were accounted by Shorea robusta, greatest WSG (0.80 g cm-3), CC (28m2) and CD (7.8m) by Hardwickia binata and largest LA (665cm2) by Sterculia urens (Table 1). In shrubs, maximum GT (24cm), HT (4m), BT (0.5cm), LA (31cm2), CC (13m2) and CD (3m) were detected in Lantana camara, highest WSG (0.7gcm-3) in Carissa spinarum and greatest LAI (3.9) in Woodfordia fruticosa (Table 2). The highest trait plasticity was observed for LA (99.2% in trees and 97.5% in shrubs) and lowest in WSG (50.0% in trees and 14.3% in shrubs) (Table 1). Lowest LA was detected in Ziziphus glaberrima (5.0cm2) at Kotwa and highest in Sterculia urens (665cm2) at Hathinala, whereas, WSG was minimum in Sterculia urens (0.4g cm-3) at Kotwa and maximum in Hardwickia binata (0.8gcm-3) at Ranitali (Table 1). Lowest LA in shrubs was observed in Ziziphus oenoplea (0.8cm2) at Kotwa and highest in Lantana camara (31.4cm2) at Hathinala (Table 2). WSG of shrub species was minimum in Lantana camara (0.6gcm-3) at Hathinala and maximum in Carissa spinarum (0.7gcm-3) at Harnakachar (Table 2). The relationships of mean value of morphological traits across all woody species at the five study sites with the respective mean value of soil properties were mostly significant (Figure 1). Height (HT) of the plant species showed strongest relationship with clay content (R=0.98, P<0.01). Soil properties such as bulk density (BD), pH and sand were negatively related with HT, whereas, SMC, C, N, P and clay were positively related with HT (Figure 1). Strongest relationship of BT was with sand (R=0.91, P<0.05). This trait was positively related with BD, pH and sand content but negatively related with SMC, C, N, P and clay (Figure 1). WSG showed negative relationship with SMC, pH, P and clay, whereas, positive relationship with BD. Positive relationship of LA was detected with SMC, P and clay content but negative relationship with BD, pH, C, N and sand (Figure 1). Plant crown cover (CC) was positively related with SMC, C, N, P and clay but showed negative relationship with BD, pH and sand. Strongest relationship of CD was observed with BD (R=-0.99, P<0.01), which was significantly negative (Figure 1). The other soil properties showing negative relationship with CD were pH and sand, but SMC, C, N, P and clay showed positive relationship with CD. Leaf area index (LAI) showed strongest relationship with SMC (R=0.96, P<0.05) as compared to other soil properties. SMC, C, N, P and clay were positively related with LAI, whereas, BD, pH and sand showed negative relationship with LAI (Figure 1).
Trait |
Min |
Max |
Mean |
Plasticity |
GT (cm) |
36.5 (Ziziphus nummularia, HK) |
104 (Shorea robusta, HN) |
64.7 |
64.7 |
HT (m) |
4.10 (Ziziphus nummularia, HK) |
16.7 (Shorea robusta, HN) |
7.80 |
75.4 |
BT (cm) |
0.60 (Anogeissus latifolia, HN) |
1.80 (Shorea robusta, GG) |
1.20 |
66.7 |
WSG (g cm-3) |
0.40 (Sterculia urens, KT) |
0.80 (Hardwickia binata, RT) |
0.60 |
50.0 |
LA (cm2) |
5.00 (Zizyphus glaberrima, KT) |
665 (Sterculia urens, HN) |
155 |
99.2 |
CC (m2) |
9.40 (Flacourtia indica, GG) |
28.4 (Hardwickia binata, RT) |
16.0 |
66.9 |
CD (m) |
1.80 (Flacourtia indica, HN) |
7.80 (Hardwickia binata, RT) |
4.20 |
76.9 |
LAI (m2 m-2) |
2.80 (Gardenia turgida, HN) |
14.0 (Shorea robusta, HN) |
7.60 |
80.0 |
Table 1 Range of morphological traits of tree species (n=40) across the study sites. GT, girth; HT, height; BT, bark thickness; WSG, wood specific gravity; LA, leaf area; CC, crown cover; CD, crown depth; LAI, leaf area index; HN, Hathinala; GG, Gaighat; HK, Harnakachar; RT, Ranitali; KT, Kotwa. Source: Chaturvedi1
Trait |
Min |
Max |
Mean |
Plasticity |
GT (cm) |
7.50 (Grewia hirsuta, RT) |
24.4 (Lantana camara, HN) |
15.6 |
69.3 |
HT (m) |
0.80 (Grewia hirsuta, RT) |
3.70 (Lantana camara, HN) |
1.50 (±0.3) |
78.4 |
BT (cm) |
0.30 (Ziziphus oenoplea, KT) |
0.50 (Lantana camara, HK) |
0.41 (±0.02) |
40.0 |
WSG (gcm-3) |
0.60 (Lantana camara, HN) |
0.70 (Carissa spinarum, HK) |
0.61 (±0.02) |
14.3 |
LA (cm2) |
0.80 (Ziziphus oenoplea, KT) |
31.4 (Lantana camara, HN) |
6.72 (±5.6) |
97.5 |
CC (m2) |
0.70 (Grewia hirsuta, GG) |
12.9 (Lantana camara, HN) |
3.20 (±1.3) |
94.6 |
CD (m) |
0.60 (Grewia hirsuta, HK) |
3.10 (Lantana camara, HN) |
1.20 (±0.2) |
80.6 |
LAI (m2 m-2) |
1.20 (Grewia hirsuta, HN) |
3.90 (Woodfordia fruticosa, HK) |
2.81 (±0.4) |
69.2 |
Table 2 Range of morphological traits of shrub species (n=4) across the study sites. GT, girth; HT, height; BT, bark thickness; WSG, wood specific gravity; LA, leaf area; CC, crown cover; CD, crown depth; LAI, leaf area index; HN, Hathinala; GG, Gaighat; HK, Harnakachar; RT, Ranitali; KT, Kotwa. Source: Chaturvedi1
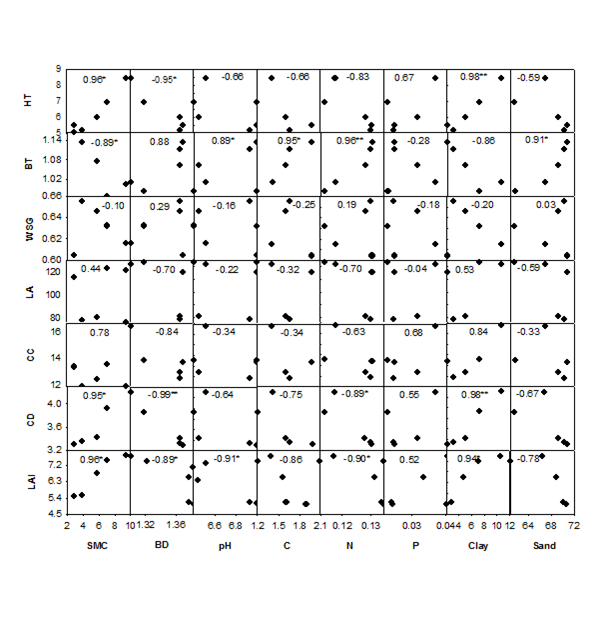
Figure 1 Relationships of morphological traits and soil properties of the five study sites. HT, height; BT, bark thickness; WSG, wood specific gravity; LA, leaf area; CC, crown cover; CD, crown depth; LAI, leaf area index; SMC, soil moisture content; BD, bulk density; C, organic carbon content; N, total nitrogen content; P, total phosphorus content; *P < 0.05; **P < 0.01. Numbers indicate R values. Source: Chaturvedi1
The morphological traits in our study are known to affect the performance of woody species either directly or indirectly.10‒16 However, the intensity of their impact is determined mostly by environmental parameters, and in tropical dry forest, soil water availability has been reported as the most important factor,19‒22 Step-wise multiple regression indicated that among the eight morphological traits, 56% variability in species richness (SR) was only explained by LA, while LA and HT together explain 65% variability in SR of the woody species. The model developed by step-wise multiple regression was. The variables in the model represent quantity of photosynthetic surface and water use economy of a species.
In the extremely variable and severe environment of tropical dry forest, the phenotypic plasticity of morphological traits of plant species plays an important role for their development, functioning and evolution. In our study, the woody species exhibited wide range of morphological traits. The phenotypic plasticity of the morphological traits showed remarkable variation across species as well as within a particular species growing at different levels of soil moisture content. The relationships of mean value of morphological traits across all woody species at five study sites with the respective mean value of soil properties were significant. Among the selected traits, LA and HT were most important for the variation in SR at our study sites. We suggest extensive investigation of the phenotypic plasticity of the morphological traits of woody species in tropical dry forest for proper management and sustainable development of the forest ecosystem.
RKC thanks Council of Scientific and Industrial Research, India (award no. 09/13(452)/2012-EMR-I) and Natural Science Foundation of China (NSFC), Chinese Academy of Science, China (grant No. 31750110466) for financial support.
The authors declare there is no conflict of interest.

©2018 Chaturvedi, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.