International Journal of
eISSN: 2576-4454


Research Article Volume 6 Issue 1
1Department of Community Medicine, Environmental Health Unit, Faculty of Clinical Sciences, Niger Delta University, Wilberforce Island, Bayelsa State, Nigeria
2Department of Environmental Health Sciences, School of Health, Allied and Environmental Science, College of Pure and Applied Sciences, Kwara State University, Nigeria
3Weather Forecasting Services, Nigerian Meteorological Agency Abuja/Department of Geography and Environmental Management, University of Port Harcourt, Choba Town, Rivers State, Nigeria
4Department of Environmental Management and Toxicology, Kwara State University, Malete, Kwara State, Nigeria
Correspondence: Olalekan Morufu Raimi, Department of Community Medicine, Environmental Health Unit, Faculty of Clinical Sciences, Niger Delta University, Wilberforce Island, Bayelsa State, Nigeria
Received: January 21, 2022 | Published: February 11, 2022
Citation: Raimi OM, Sawyerr OH, Ezekwe CI, et al. Many oil wells, one evil: comprehensive assessment of toxic metals concentration, seasonal variation and human health risk in drinking water quality in areas surrounding crude oil exploration facilities in rivers state, Nigeria. Int J Hydro. 2022;6(1):23-42. DOI: 10.15406/ijh.2022.06.00299
Background: Oil and natural gas extraction have produced environmental pollution at levels that affect reproductive health of indigenous populations. Accordingly, polluted drinking water from physical, chemical and heavy metals can result in serious health problems, like anemia, kidney failure, immunosuppression, neurological impairments, gastrointestinal as well as respiratory irritation, skeletal system abnormalities, liver inflammation, liver cancer, cardiovascular diseases after chronic exposure and other cancer diseases with negative health effects. These diseases types remain associated to high amounts of heavy metal elements such as lead, chromium, zinc, copper, cadmium, manganese as well as nickel etc.
Objectives: Compare differences in water quality parameters in the study area (determine the level of pollutions in the different sites).
Methodology: The investigation made use of standard analytical methodologies. All sampling, conservation, transportation as well as analysis followed the usual APHA procedures (2012). To prevent degradation of the organic substances, all obtained samples were transferred to the laboratory, while keeping in an icebox.
Results: Result shows that during wet season, the mean values obtained for water quality parameters were significantly lower in site 9 compared with that obtained in other sites (p<0.05) with the exemptions of temperature, DO, BOD, COD, acidity, TH, TDS, K, Mg, Zn, Mn, Cd, Pb, Cu, Cr, NH3, NO2, NO3, Ni though slightly lower in most cases in site 9 were not significantly different (p>0.05) and both alkalinity and SO4 which were significantly higher in site 9 than site 1 (p<0.05). Result obtained during dry season reveals that there is no remarkable difference in pH, acidity, Pb and Ni between the nine sites (p>0.05) while other water quality parameters were significantly lower in site 9 than other sites excluding Cl and Mg which were both significantly higher in site 9 than site 8 (p<0.05).
Conclusion: To guarantee quality groundwater supply for various purposes in Nigeria's core Niger Delta region, extra efforts must be taken to fully understand hydrogeochemical features and its suitability. Thus, this study will aid in the development of a quantitative understanding of the effects of diverse causes on groundwater level fluctuations in any aquifer around the world. Also, this analysis reinforces a valuable resource for researchers, activists and public officials seeking to help enhance community awareness, planning and performance. The verdicts would remain a valuable guideline for policymakers, the Ministry of Water Resources and development practitioners, as this highlights the requirement for suitable approaches toward mitigating toxic element of water resources contamination in the core Niger Delta toward safeguarding health of the public from carcinogenic as well as non-carcinogenic risks.
Keywords: Human health risk, Oil wells, Reproductive health, potentially toxic metals, Development practitioners, Community awareness, Exploration Facilities, Core Niger Delta.
The total worldwide fuel consumption of oil as well as gas accounts for roughly 56%. About 100 million barrels of oil as well as 10 billion cubic metres of natural gas are produced daily by the industry. Thus, from operations of the oil as well as gas, discovery through manufacturing, storing as well as shipping goods, may have a significant environmental influence.1 Consequentially, it's possible that it will have an effect on human health, biotic as well as abiotic or cultural heritage sites on a local or regional level.2–8 While, the area plays host to over 100 oil wells, 9 gas flaring sites, an oil and gas gathering centre, a gas plant, a gas to power plants and several hundreds of kilometers of oil and gas pipelines all located within an area of less than 250 km2.9–14 Niger Delta region has a difficult terrain, and as such all kinds of environmental pollution is quite high.11,4,12 Water bodies in the Niger Delta have been heavily polluted due to recurring incidence of oil spillage.15 Most micro-populations following several large-scale oil spillages have generally affected aquatic resources.16 As a result, freshwater resources contamination has come to be an issue of worldwide concern. It has been put forward that it is the largest cause of fatalities as well as diseases, with over 14,000 individual’s deaths daily.17–23 In spite of the about US$600 billion generated from oil revenue between 1958 and 2007, Nigeria has little or nothing to show in terms of social amenities and infrastructural provision.24 Groundwater is rapidly being exploited for drinking-water supplies, according to studies, partly due to dwindling water resources scarcity as well as because groundwater is valued as a source of “clean” drinking water. Ground water accounts for around half (50%) of the potable water supply in Nigeria. Approximately 80 percent of homes in Niger Delta rely on this domestic source of water supply. Concern about groundwater supply safety have focused on pollution caused by human activities, with contamination from natural origins receiving less attention.25 The absence of safe water is linked to this, which exacerbates health problems and has a negative impact on productivity. Only around a quarter (24%) of the indigenous population as well as half of the urban population in the Niger Delta have access to drinkable water, according to the UNDP (2006).26 This is in accordance with the findings of a Bayelsa State Micro Credit Administration Agency poverty baseline survey, which indicated that only a tiny percentage of the indigenous populace has access to drinkable water. However, several studies9,10 have shown and document (scientifically) that increasing presence of geogenic contaminants in the Niger Delta can have serious health effects as well as wellbeing on the indigenous population, thus leading to both environmental and community concerns, resulting in oil and gas companies’ prohibition in some locations. The volume as well as frequency of hydrocarbon spills larger than one barrel (bbl) that reach the environment remain reported as normal industry practice. For reference, 1 barrel equals 42 gallons or 159 litres in the United States. As a results, Figure 1 below illustrates some of the most important sustainability challenges for the extractive industry. It likewise demonstrates how social, economic as well as environmental factors are all inter-connected in nature. This covers a wide variety of positive as well as negative impacts that companies may face while addressing sustainability risks as well as opportunities.
While a variety of harmful compounds, for example, cadmium, TPH and other chemicals can be discharged into the environment as a result of oil and gas companies’ operations. Many of these contaminants have been proven to be harmful to human health. Exposure to cadmium as well as hydrocarbon (benzene) is classified as very carcinogenic for humans by the International Agency for Research on Cancer. Additionally, groundwater pollution is probable to contain very harmful substances resulting from industrial production, such as lead, prior epidemiological research has indicated that main lead health outcomes include cancer as well as congenital malformation and have been found to be statistically associated with exposure to groundwater pollutions.9,10,4,13 Arsenic, chromium, fluoride, barium, boron, selenium as well as uranium are all common geogenic chemicals that have health implications in drinking water,27 nonetheless arsenic (As) as well as fluoride (F) have received the maximum attention owing to their toxicity as well as widespread distribution.28 The geochemical features of the aquifer material, especially significant concentrations of the pollutant within the rock matrix may cause geogenic contamination of groundwater. This dissolves as a result of water-rock contact and/ or environmental conditions, making the pollutant more mobile. The aquifer type (whether unconfined or confined) as well as the depth of water table are two factors that influence naturally occurring pollutants. Unconfined aquifers remain more prone to pollution than confined aquifers, according to Berardinucci and Ronneseth (2002), since groundwater is younger as well as moves through the system more quickly. Surface derived contaminants remain more sensitive in aquifers with shallow water tables. Over 200 million individuals globally, according to UNESCO estimates, rely on drinking water that contains trace elements concentrations in amounts that exceed the current WHO (2017)29 recommendation (equal to the population of Nigeria, thus detrimental to life expectancy than COVID-19 in Nigeria). At the local, regional and national levels, access to clean drinking-water is a critical health as well as development concern.27,19,30,20,21 Controlling drinking-water quality necessitates the growth of management strategies that involve a drinking water assessment system toward identifying potential dangers. As groundwater becomes a significant supply of freshwater for residential use in the Niger Delta along with most Nigerian cities, it is critical to examine its quality assessment, particularly in terms of geogenic pollutants. This is because people's reliance on groundwater from shallow aquifers puts a large number of individuals at contamination risk to geogenic sources. Trace elements remain amongst the few chemicals that have remained demonstrated to have extensive health problems in humans as a result of excessive amounts of exposure by drinking-water, whereas iron is important due to its acceptability effects. As a consequence, towards addressing these concerns, the goal of this research paper is to compare differences in water quality parameters in the study region (determine the level of pollutions in the different sites) in the vicinity of “Gas Flaring Area of Ebocha-Obrikom of Rivers State, Nigeria”. Thus, this research will provide valuable information and add to our understanding on the physico-chemical examination of drinking water associated with the contamination of the ground waters by petroleum products. Hence, the ground water quality in the study area will be highlighted in this study: it will also bring to the awareness of the local people the type of water that is good for them as drinking water according to recommended standards. Also, it will provide a structural framework for effective and accurate management of groundwater and provide an available reference source and base line data for researchers involved in water resources assessment; thereby, contributing to the existing literature. The study will help government in policy intervention and supervision for better health service delivery. Furthermore, it will help in integrating the health needs of the populace into the state health scheme, in recognition of the fact that health is required for national development. Besides, it is expected that the result of this study will assist toward meeting the challenges of achieving improved community health status in the state, strengthen the already existing strategies toward providing good quality water in Rivers state and Nigeria at large.
Pollution from groundwater has been globally documented as a foremost threat to public/environmental health, as well as it obstructs the United Nations Sustainable Development Goals (SDGs) accomplishment, together with those associated to poverty eradication (SDG 1), zero hunger (SDG 2) as well as good health and wellbeing (SDG 3), ensure access to clean water and energy, address needs for quality education and tackle climate change, and protect life on land and in the oceans. They address humanity’s challenges, and are a universal call to protect the planet and improve lives. To support this process, drinking safe water is a basic necessity for human development, health as well as well-being, and hence, an internationally recognized human right and has long been a goal of national and international policy.20,21 Pollution from groundwater disproportionately affects the most disadvantaged, particularly children as well as women (SDG 5).31,32,13 Contaminants leaching into groundwater as well as runoff threatened the availability of safe drinking water (SDG 6) (Table 1). The Sustainable Development Goals (SDGs) of the United Nations comprise ambitious worldwide targets for drinking water, sanitation as well as hygiene. The SDG indicator for target 6.1, usage of safely managed drinking water services (SMDW), attempts to rectify the shortcomings of prior monitoring efforts. Improved drinking water sources (protected groundwater sources, piped water, rainwater collection as well as packaged or delivered water) that remain available on premises, accessible when required as well as free of contamination are described as SMDW services. Thus, the need to increase drinking water quality monitoring in Nigeria's Niger Delta Region is widely recognized.9 Oil and Gas industry domicile in the area may be positioned to include water quality monitoring in their activities; as it is remarkable that water safety activities do not compromise. While, the UN Sustainable Development Goals (SDGs) remained introduced in 2015 as a broadly agreed, wide-ranging plan of action aimed at environmental sustainability, social inclusion, as well as economic development. Because the extractive industry plays such a significant role in the universal economy, the SDGs are primarily targeted at governments. The 17 Sustainable Development Goals (SDGs), defined by the United Nations (UN) in its 2030 Agenda for Sustainable Development offer a framework/roadmap aimed at creating a better future for people as well as the planet come 2030. The SDGs were adopted by all United Nations Member States in the year 2015 and remain a call to action for all countries – rich, middle-income and poor, to increase prosperity while safeguarding the milieu. They understand that water is a vital resource for agriculture, human development, industry and that it can contribute to economic progress and meet a variety of societal demands.33 The United Nations (UN) considers water access as well as sanitation to remain a human rights problem, with everyone entitles to adequate, acceptable, safe, physically-accessible and inexpensive water for personal as well as household purposes.20 Demand for freshwater resources is anticipated to intensify as the world's population grows, urbanization accelerates together with agricultural and economic development.34–39 Changing climate, land use, as well as water availability, reliability and quality are just a few of the concerns that could have an impact on the activities of the oil as well as gas industry.40–52 For example, industry operators might consider locating operations in areas where water supply and quality are presently or may become a problem in the future, or in areas prone to extreme weather as well as flooding.53 Water scarcity can have a remarkable consequence for local populations as well as stakeholders.54 Industrial users, such as the oil and gas industry, may face physical, regulatory as well as reputational issues as a result. As a result, the International Petroleum Industry Environmental Conservation Association (IPIECA) and others collaborated an Atlas that depicts the oil and gas industry contribution to the SDGs, with all SDGs potentially relevant. Figure 2 depicts the guidance modules, which provide particular information that can be used to illustrate SDGs contribution.

Figure 2 Mapping the modules to the SDGs Adapted from Global Reporting Initiative and United Nation (2015).
Hence, the complexity of the SDGs means that many of the goals need to be addressed through social, political and financial changes. Science and technology are vital to this process, according to the 2016 Global Sustainable Development Report (https://sustainabledevelopment.un.org/content/documents/10789Chapter3_GSDR2016.pdf). Advances in nanotechnology could help improve battery storage or water filtration; developments in Earth sciences could inform natural resource policy; and breakthroughs in medicine could impact healthcare. Addressing the SDGs therefore requires interdisciplinary research: collaboration among natural and social scientists, policymakers, and industry leaders.
From extraction through end-product supply, the oil and gas sector comprise a wide range of activities. The value chain is the term used to describe this spectrum. Figure 3 depicts the kind of "activities that a fully integrated oil and gas" corporation with extensive downstream as well as upstream operations might engage in. Thus, an oil and gas activities from companies at a particular area can span decades, for example, from early offshore exploration to platform decommissioning. Hence, there is need for companies to be encouraged to evaluate the effects of their actions across the various value chain.
While, the Niger Delta region of Nigeria produce majority of the nation’s oil and gas, producing 85 million estimated barrels of oil equivalent daily. Despite the fact, oil companies operating in the Niger Delta have an impact on how millions of individuals gain (or suffer) from the country’ hydrocarbon reserves. These corporations oversee “multi-billion-dollar portfolios of public assets”, carry out complicated projects across their territories as well as at sea, employ tens or hundreds of thousands of individuals, as well as provide a variety of public services ranging from energy generation to infrastructure building. Regardless of their relevance, flaring of natural gas and oil spills may have severe as well as multiple environmental, health, social as well as economic effects on a company’s reputation and indigenous communities.5 Oil spills, which are likely to occur as a result of operational incidents, inadequate maintenance, or corrosion of equipment, can have serious as well as widespread environmental, social, health and economic effects in the worst-case scenario.44 Spills can also have serious long-term ramifications for a company’s reputation. The health risks and impacts from oil gas industry resulting from groundwater pollution remain linked to some contaminants (or hazardous substances) created by physical-chemical interactions. Contaminants can travel through the soil, air as well as water in general.18 They can likewise settle on or be digested through animals or plants and they can end up in the food chain, air as well as water.55 The various ways in which an individual can come into contact by means of contaminants remain referred to as exposure pathways. There are three primary routes of exposure, namely: ingestion, inhalation as well as skin contact. Inhalation is the act of breathing or inhaling into the lungs. Ingestion is the act of ingesting something via mouth. When anything comes into direct contact with the skin, this is referred to as skin contact. After skin contact, ingestion can be a secondary exposure pathway. Acute or chronic exposures can occur. An acute exposure is defined as a single brief exposure to a harmful material (pollutant). Health symptoms may emerge quickly following exposure; for example, a gas release is hazardous because to the risk of fire and explosion, whereas an oil release might have very different and potentially catastrophic implications for the environment and individuals. Thus, leading to a cause, life loss, or a serious fire. Prolonged exposure happens over a significantly longer length of time, frequently with smaller amounts of exposure in repeated ways. People living near Love Canal,56 a leaking hazardous waste dump, for instance, did not recognize the health repercussions of prolonged exposure for some years. Chronic health impacts are often long-term illnesses or injuries like liver failure, cancer, stunted growth as well as development. Bioaccumulation is one of the mechanisms by which persistent exposure to even trace levels of harmful substances can cause harm. Some compounds are absorbed rather than expelled and remain in the human body. They build up and cause harm over time. As a result, detrimental health impacts are determined by the determinants of exposure. The following factors influence whether or not unfavorable health impacts may occur as a result of an exposure:
The entire procedure of health risks assessment and the groundwater impacts is quite challenging and necessitates a great deal of expertise, time as well as financial resources to complete. Its successful implementation necessitates dealing with the lack of exact data on the dosage response association for some of the concern chemicals, as well as making a variety of educated assumptions as well as interpretations in order to gain an improved understanding of what is more or less essential. For the study implementation, environmental samples (water and soil) need to be examined toward determining the content as well as several pollutants concentrations (polychlorinated biphenyls, heavy metals and pesticides) known to be harmful to human health. Groundwater samples collected from the gas flaring site were compared to samples collected from another location, a peri-urban residential area approximately 30km away from Ebocha-Obrikom oil and gas field. Below, is the flow chart (Figure 4) of the study, highlighting the relationship amongst the environmental contaminants from the groundwater pollution as well as environmental/public health impacts on the indigenous and surrounding communities. This flow chart, which shows the conceptual relationship between health and groundwater pollution, is typical of any similar investigation. One of the most critical variables that determine the public/environmental health dangers of groundwater is the waste streams disposed of in it.
The study area
Ebocha-Obrikom is located amongst latitude 50 20N - 50 27N as well as longitude 60 40E - 60 46E (Figure 5). It includes the towns of Obor, Obie, Obrikom, Agip New Base and Ebocha, all of which are positioned in Ogba/ Egbema/Ndoni Area (Figure 5) of Rivers State. The research study area is bordered to the North by the Nkissa River, to the West by the Orashi River, to the East by the Sombrero River, and to the South by Omoku town.32
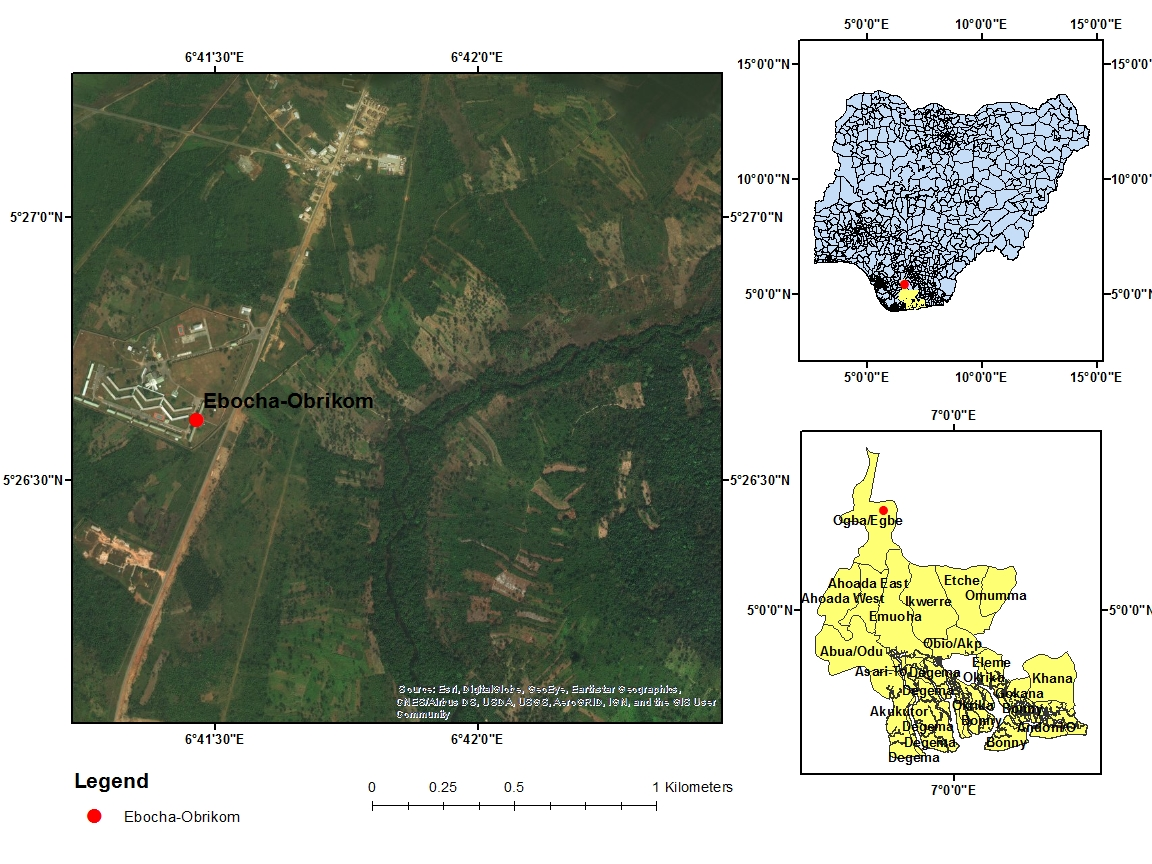
Figure 5 Map Showing the Study Area with Nigeria and River State insert.
Sources: Adapted from Olalekan et al., 2018b [https://doi.org/10.4236/ojogas.2018.33017].
Climate
The study area has an equatorial type of climate. The region receives approximately 2500 mm of rain every year. Rainfall through the year, occurs for eight (8) months (March to October), as well as even months termed dry are frequently not devoid of sporadic precipitation.14,19 Temperatures are often high, with the average monthly temperature hovering around 25 degrees Celsius.
Vegetation
Fresh water marshes dominate the vegetation, with spotted sections defined by stratified high forest: The vegetation consists of a plethora of evergreen trees that provide tropical hard wood, such as mahogany. Smaller palm trees remain abundant, as are ascending plants like lianas and rattan palms, which can grow to be hundreds of meters long, as well as epiphytes and parasites that live on other plants. Below the trees, a plethora of orchids, creepers, and ferns thrive.19–21 In most cases, native rainforest flora has been replaced by towns, farms, fallow as well as secondary forests, civic structure development, and oil and natural gas exploration/exploitation. The only sites where primary forests can remain found include riparian woods dominated by raffia palms along the Sombreiro River, across the Orashi River, and holy groves, which are relics of traditional African religion.19–21
Topography and drainage
The territory is essentially drained via the Sombreiro on the Eastern flanks as well as Orashi on the Western flanks, resulting in an essentially continuous inter-basinal area. The area has a nearly flat topographic structure and is covered via superficial soil composed of silty clays and silty sand. There is a distinct lack of significant hills rising over the entire land surface to a height of around 25 metres above sea level.57 The River Orashi is a key component of the natural drainage system. Despite being a separate river system that drains the entire zone, this river connects with the Niger Delta system during the wet season (flood stage). Because of the more obvious relief of this study area, drainage is more efficient and the area is served by fewer rivers and creeks. There are backswamp depressions that entrap floodwaters and generate perennial lakes in the area. Rivers are prone to floods, which raises the water level in the water table. Tidal flow affects the rivers in the research area as well.20,21,57
Soil
The soil in this location is part of the Niger Delta current alluvium as well as it exhibits differences in age in the development of the Argillic layer (clay illuviation). On top, they remain predominantly sandy loam, shifting to sandy clay loam as well as eventually clay in the subsoils. The reaction in the soil is acidic (pH 4.3-5.0).58 The high soil acidity is caused by the soil's high Aluminium (Al) concentration.17,59 The organic matter level of the soil is also low, which could be attributed to the land's ongoing agriculture. Organic matter reduces as one moves down the soil profile. Phosphorus (P) availability is moderate, particularly in the middle horizons. Soil fertility is high in terms of percent base saturation. Alfisols (Luvisols and Natrosol), Ultisols (Regosols) as well as Oxisols (orthic Ferralsols) are the most common soil types. Other soils discovered include inceptisols (Gleyic cambisols) as well as entisols (Albic Arenosols) are two further soil types identified. Ultisols (Eutric-Paleudalfs) are the soils.60
Land use
The land use patterns in the Ebocha-Obrikom area are diverse. Crude petroleum exploration and exploitation remain the only observable economic activity in the surroundings. The area's land use pattern is primarily defined by a variety of agricultural activities, including fishing and farming, which remain among the principal activities. Cassava, cocoyam, yam, bitter-leaf, okra, and other crops are grown. Oil palm planting, harvesting, as well as processing remain also critical.20,59
Sample collection
The current research inquiry used a sample method similar to that used by Morufu and Clinton9 Raimi and Sabinus,10 and11 in which sampling was targeted in selected vulnerable quarters in a highly populated environment. These areas are prone to pollution not just due to their physical location, but also due to the existence of crude petroleum exploration and exploitation. Water samples were taken from groundwater sources near the sampling location (Table 2) and used mostly for drinking and domestic purposes. Only groundwater from dug wells or shallow pumping wells built specifically for residential use was sampled. The wells range in depth from 10 to 28 meters, indicating that they are in a phreatic aquifer. Portable GPS devices were used to record the sampling locations. Ground water sources in the neighborhood of the depot were chosen at random but at varying distances from one another for the purposes of this experiment. Furthermore, samples were manually collected from nine (9) strategic locations in the study area for ground water (boreholes and wells) into previously washed clean plastic sampling bottles after approximately 20 minutes of continuous water flow to ensure adequate aquifer that can remain suitably represented.
Groundwater samples were collected once a month from well and borehole across the nine sites. All samples were collected during the day, from 9:00am to 4:00pm. As a result of flooding, insecurity as well as lockdown from COVID-19. Night samples were not taken, and the sampling took place between the month of September 2019 and August 2020. The depth ranged from 10 to 28 meters.
Sampling, preservation and analysis
Water sampling, conservation, transportation, and analysis have all been carried out in accordance with the standard methods specified in APHA.61,9
Ground water collection
Ground water samples were obtained in pre-rinsed 1litre plastic containers for analyses of physico-chemical characteristics. Prior to storage, pre-rinsed ground water samples for trace metal analyses remained obtained in 1litre containers with nitric acid and treated with 2ml nitric acid (assaying 100 percent, Fisher Scientific, Trace Metal Grade). These steps were taken to keep the metals oxidation settings stable. For Dissolved Oxygen (DO) and Biological Oxygen Demand (BOD) assays, groundwater samples remained obtained in two groups of 250ml glass-stoppered-reagent bottles per sampling site. The BOD samples were carefully filled without air trapping and the bottles were wrapped in black polythene bags. This was done to exclude the presence of light in the samples, which was capable of creating DO by autotrophes (algae). The BOD samples were cultured for five days before being added to 2ml of each sample. Winkler solutions I and II apply various dropping pipettes to each sample to slow down additional biological activity. To precipitate the floc that was at the bottom of the bottles, the bottles were thoroughly shaken. Furthermore, Winkler solution I is a manganese sulphate solution, whereas solution II is a mixture of sodium or potassium iodide, sodium azide (sodium nitride), sodium or potassium hydroxide as well as sodium hydroxide. The samples of DO were collected in transparent bottles with tight-fitting stoppers. With dissolved oxygen samples kept on the spot using Winkler I and II solutions identical to those used for BOD samples (APHA, 2012).61 For simple identification, all samples had remained carefully identified and kept at 4°C. On-site measurements were taken to determine the unstable concentrations and sensitive water quality indicators such as pH, electrical conductivity (EC), total dissolved solids (TDS), alkalinity (Alka.) as well as temperature (Temp). As a result, Figure 6 depicts the key methodologies for researching groundwater composition.
Quality assurance and quality control (QA/QC)
Furthermore, using high purity analytical reagents and solvents, all analytical methods remained closely monitored using quality assurance and control methodologies. The instruments were calibrated using calibration standards. The analytical technique validation included the use of triplicate analysis, procedure blanks and the examination of certified reference materials (CRM). The limit of detection (LoD), precision, reproducibility, repeatability and accuracy of each organic pollutant from the groundwater samples were determined.
Compare differences in water quality parameters in the study area (determine the level of pollutions in the different sites)
Results of site difference in water quality parameters in wet and dry seasons are presented in Table 3 and 4. Result shows that during wet season, the mean values obtained for water quality parameters were significantly lower in site 9 compared with that obtained in other sites (p<0.05) with the exemptions of temperature, DO, BOD, COD, acidity, TH, TDS, K, Mg, Zn, Mn, Cd, Pb, Cu, Cr, NH3, NO2, NO3, Ni though slightly lower in most cases in site 9 were not significantly different (p>0.05) and both alkalinity and SO4 which were significantly higher in site 9 than site 1 (p<0.05) (Table 3). Result obtained during dry season reveals that there is no significant difference in pH, acidity, Pb and Ni between the nine sites (p>0.05) while other water quality parameters were significantly lower in site 9 than other sites excluding Cl and Mg which were both significantly higher in site 9 than site 8 (p<0.05) (Table 4).
Compare differences in water quality parameters in the study area (determine the level of pollutions in the different sites)
The phrase “trace elements” refers to a class of ubiquitous elements that are common in the environment, yet are harmful to people and organisms at very low quantities. Heavy metals (metals having a large atomic mass) for instance chromium (Cr), cadmium (Cd), lead (Pb), nickel (Ni), cobalt (Co), copper (Cu), mercury (Hg), tin (Sn), as well as zinc (Zn). While non-metals that remain viewed as trace elements comprise antimony (Sb), arsenic (As), as well as selenium (Se). Trace elements remain long-lasting and are not destroyed by metabolic activities. Trace elements exist in a variety of forms, including oxides, salts, organometallic complexes, sulphides, and dissolved ions in groundwater as well as soil solution. Chemical processes are driven by the partitioning of water, air as well as soil through particles adsorption or pH-dependent water dissolution.62 Trace elements remain geogenic (natural) in origin, because several rocks comprise significant quantities of trace elements that remain released into the milieu via anthropogenic or weathering action. Thus, Table 3&4 provide the statistical analysis findings for the physicochemical parameters. The Ebocha-Obrikom area of Rivers State is significant for irrigation, drinking, as well as industrial uses. Over the previous three decades, the Ebocha-Obrikom area of Rivers State has been significantly altered by population expansion as well as increased agricultural productivity. However, various small-scale entrepreneur activities and anthropogenic activities such as extractive industries and it related activities, as well as chemicals overuse in agriculture remain hypothesized toward degrading groundwater quality in Ebocha-Obrikom area of Rivers State and toxic element contamination of the food chain. The groundwater investigation of toxic materials in the Ebocha-Obrikom area of Rivers State is consequently vital for human health protection. A detailed analysis of groundwater geochemistry as well as associated estimation of community’s health risk that are visible to the groundwater, remain yet to be carried out, despite the fact that a clear understanding of the utmost significant aspects regulating the health risks is vital towards taking effective management measures for the residents regarding drinking water. Thus, the concentrations of analysed water quality parameters is summarized in Table 3&4. A total of thirty-four (34) water quality parameters were analysed during rainy and dry season respectively. Eighteen (18) parameters such as temperature, pH, conductivity, turbidity, DO, BOD, Acidity, TSS, Salinity, Fluoride, Aluminum, Potassium, Magnesium, Iron, Zinc, Manganese, Cadmium and TPH were lowest at location nine (9) during the rainy season.
For dry season, twenty-two (22) parameters, which include: turbidity, BOD, Acidity, TH, TDS, TSS, Salinity, Fluoride, Aluminium, Potassium, Calcium, Iron, Zinc, Manganese, Cadmium, Copper, Chromium, Sulphate, Ammonia, Phosphate, Nickel and TPH recorded minimum values below limits of detection in sampling locations nine (9). Thus, the results showed a significant disparity between the various sampling locations. As it is evident that samples from location 1 to location 9 must remain adequately observed since, there may be a remarkable increase in these heavy metal level in the future, which could eventually cause health-related threats for indigenous residents. The temperature is one of the utmost critical elements influencing the biological activity of an aquatic organisms. Discern that there is high temperature variation in this region. Temperature was found to be highest with an average value of (28.77–32.46)0C at location 4 and 2 during the rainy and dry seasons. While it was lowest (26.01–29.00) 0C at location 9 and 6 during the rainy and dry season. The maximum permissible limit for temperature has not been stated but ambient in nature. Groundwater temperature tend to be influenced more by dry season than rainy season. Thus,9 indicated that locations predisposed to the release of industrial pollutants and gas flaring typically have temperatures that are higher than those of their surrounding environments. Unarguably, the gas flare site must have influenced an increase in air temperature, thus correspondingly increasing the temperature of groundwater. This is indicative of groundwater pollution since anthropogenic activities seem to have influence groundwater pollution more in the dry season. Likewise, rising temperature may adversely impact agriculture, thereby increasing the vulnerability of marginalized agriculture-dependent rural populations of “Ebocha-Obrikom Community in Ogba/Egbema/Ndoni Local Government Area of Rivers State, which is home to Agip’s Ebocha, Obrikom as well as Obiafu Oil and Gas Facilities”. Thus, gas flare site can be a catalyst for such migrants to relocate in search of better living conditions and alternate livelihoods. Despite, the fact that the community is abundant in natural gas as well as is home to the state-owned gas turbine facility. There are several large and functioning gas stacks in the community.
The people of Ogba remain primarily fishermen as well as farmers who rely on groundwater and small streams particularly the Orashi River as community water source. Water pH represents hydrogen ion concentration as well as is affected by the interaction of several compounds dissolved in water, aquatic animal respiration, photosynthetic activity of aquatic plants, as well as organic matter decomposition. The natural water pH influences biological as well as chemical interactions, and controls the metal ions solubility as well as the consequences on natural aquatic life. The pH range in which aquatic creatures thrive varies. It is in itself poisonous at a certain level as well as been shown toward influencing the toxicity of heavy metals, cyanides, hydrogen sulfide as well as ammonia. Even though the pH value of water still remains a significant indicator of its acidity or alkalinity. Water hydrogen ion has no direct detrimental influence on consumers, as well as an indirect outcome, but it can cause changes in several characteristics of water quality, such as the primary chemical formation as well as survival of infectious microorganisms in water. Aside from triggering irritation of the digestive tract in persons with high sensitivity.63 The pH has no direct impact on human heath, it can change water taste as well as exhibit linked to some other water quality characteristics. The carbonate cycle composed of CO2, H2CO3, HCO3- and CO3-2, which plays a role for regulating pH. The importance of alkalinity lies in its role for carbon dioxide chemistry, trace metal speciation and buffer capacity of the groundwater. Thus, hydrogen-ion-concentration (pH) is a master control measure in the environment that demonstrates the chemical as well as biological features of water as well as it is used in identifying waters alkalinity or acidity. It is remarkably critical to understand the water nature, as well as it also reveals a close relation with other water chemical constituents.
The main variables affecting the variation of pH in any milieu are: dissolved oxygen, water temperature, land runoff, decomposition of organic matter photosynthetic activity respiration of aquatic organisms and some physic-chemical processes, such as precipitation and oxidation reduction tacking place in the environment. All of the aquifers in the rainy season contained groundwater that had slightly acidic to slightly alkaline nature of groundwater, with on-site pH values ranging from 5.82 to 7.98. The highest pH was found in location 2, possibly due to more intensified human activity in such location and the lowest was detected in location 9, indicating that gas flaring at Ebocha-Obrikom area of Rivers State seems to have affected groundwater acidity. Albeit the waters may not show the apparent threat to human health dependent on pH, they would be destructive to picturesqueness after some time.9,64 Similarly, the analytical results during the dry season discovered that pH ranged from 5.99 to 7.23, which was within the WHO (2017) acceptable pH range of 6.5 to 8.5 for drinking water, except at sampling location 1, which had the highest pH value. Thus, on pH scale, a number of 7 denotes neutral water, a value less than 7 denotes acidic water, and any value greater than 7 denotes basic water. Hence, the increasing pH values could lead to increase in the rate of corrosion. In any of the groundwater tests, nonetheless, no location was determined to remain beyond the maximum permissible limit.
The pH ground water variation in Ebocha-Obrikom area was below the permitted level and was therefore, not hazardous for drinking. Electric conductivity (EC), is a measure of an ion's capacity toward carrying electric current in a solution.54 This capacity is affected by ions presence, mobility, their total concentration, valence, relative concentration as well as temperature. The higher the conductivity, the warmer the water. The existence of inorganic dissolved solids like nitrate, sulfate, chloride as well as phosphate anion or calcium, sodium, iron, magnesium, as well as aluminum cations affects water conductivity. Oil, alcohol, phenol, as well as sugar are organic compounds that do not transmit electrical current well and so have low conductivity in water. According to Kanga et al., (2020), the EC is often used to calculate the ionic concentration of groundwater, which fluctuates based on the concentration, ions type presents in water, as well as temperature. The most important test that reveals the total concentration of soluble salts is the conductivity test. Thus, electrical conductivity (EC) diverges from (24.2 -52.29)(11.93–46.76)μs/cm, with an average of (31.83–26.81)μs/cm (Table 3&4). Because it is a measure of a material’s capacity toward conducting an electric current, the disparity suggests a wide range of salts existing in groundwater. TDS is indicated in terms of the degree of salt concentration in the studied area. Thus, the percolation of agrochemicals and natural groundwater recharge processes increases the EC value.65 Hence, higher values for conductivity at location 2 & 4 could be attributed to excessive accumulation of dissolved salts, spilled oil through possible emission of flared gases getting into groundwater, agricultural lands and other organic materials present as natural resources. Equally, salinization could affect quality of groundwater in the research study area in the following years.
However, gas flared environment samples remain a comparatively more conductive. In the vicinity, the salt water infiltration effect into the aquifer from the tides affected by river Orashi may possibly play a role in the salinization of groundwater in the area.66 The result found support in Ehirim and Nwankwo67 which established that electrical conductivity values of the ground water samples collected from the studied location are observed to be low throughout the sampling locations and the variations of their mean concentrations at different distances. According to Okafor and Opuene68 the electrical conductivity indicates the level of salinity; hence it greatly affects water taste as well as represents a remarkable impact on the user’s acceptance. Turbidity is defined as “the optical quality of a sample of water that causes light to remain dispersed as well as absorbed rather than diffused in straight lines across the sample” by the American Public Health Association.9 Turbidity, in a nutshell, answers the query, “How foggy is the water?” The capacity of light to flow through water is related to the suspended particles volume within the body of water. The more suspended particles there are in the water, the cloudier it becomes. An electronic turbidity meter is used to measure turbidity. The results are provided in Nephelometric Turbidity Units (NTU) or by filtering a water sample as well as comparing the colour (how bright or dark it is) of the filter to a standard turbidity chart. The APHA recommends that the turbidity of drinking water not exceed 5 NTUs. As a result, high turbidity levels may raise the risk of waterborne disease. If turbidity is mostly caused by organic particles, depletion of dissolved oxygen in the water body may occur.9 Increased degrees of turbidity remain frequently related with higher amounts of pathogenic microorganisms like parasites, viruses as well as certain bacteria.26 These microorganisms can produce symptoms like cramps, diarrhea, nausea as well as associated headaches.69 Thus, highest value of turbidity was noticed at location 7 (48.24–16.75) NTU during the rainy and dry season. Groundwater turbidities remained below the usual recommended maximum tolerable limit of 5NTU for drinking water at location 6 & 9 for rainy season and location 5, 6, 8 & 9 for dry seasons. Although, location 8 (rainy season) and location 1 & 2 (dry seasons) approximately attained the maximum permissible limit for drinking water. Thus, turbidities were higher during rainy season than dry season. Hence, wet season influence turbidity more than its counterpart dry seasons. This could be owing to the research area's continual and significant tendency to receiving huge amounts of organic as well as inorganic material emitted by gas flaring as well as oil spillage contaminating the ground water.
Dissolved Oxygen studies in water remain critical because it is regarded as one of the most critical limiting variables for the life of aquatic species, influencing biological processes such as aquatic organisms, animal respiration and the oxidation of organic materials in water as well as sediments. It is a crucial metric in determining the pollution degree, as sewage pollution is widely viewed as an organic pollutant that affects fish as well as other aquatic life, mostly through oxygen deprivation. As organic matter decomposes, bacteria devour oxygen. As a result of the abundant organic debris, an oxygen deficient milieu can form in lakes as well as rivers. These settings can ultimately result in fish kills, restricted growth, life cycles disruption, migration toward avoiding unfavorable condition as well as mortality of benthic creatures.70,71,9 Thus, highest value of Dissolved Oxygen (DO) for groundwater was noticed at location 3 (17.84–19.10) mg/L for both rainy and dry seasons. It was observed that DO was higher during both seasons. Thus, indicating higher biological activity due to higher rate of anthropogenic activities. Meanwhile, studies by Chapman and Kimstach72,9 found that DO concentration below 5mg/L have a negative impact on the functioning as well as biological communities’ survival and concentrations below 2mg/L may result in the death of more life. The BOD is the quantity of oxygen required by bacteria during organic material degradation. It contains the oxygen needed aimed at the oxidation of numerous chemicals found in water like ferrous iron, sulfides, as well as ammonia.71 This parameter indicates the microbial respiration ability toward breaking down organic material in the water, which results to low DO as well as a possible cause of hypoxia.9 Even though, biological oxygen demand (BOD) reflects the amount of oxygen needed by bacteria. It is used to determine any receiver environment pollution potential as well as assimilation capacity. The present study for BOD had its highest value at location 4 & 6 (5.48–6.09) mg/L during rainy and dry seasons. Dry season had higher values than rainy season. In consequence, it might be extrapolated that anthropogenic activities may influenced increased BOD during the dry season, while supporting higher biochemical activity.
However, irrespective of seasonal variations, both seasons influenced BOD. Hence, gas flaring could have played a role in this pattern. The oxygen amount needed to oxidize organic materials in waste water using a strong oxidant as well as transform it to carbon dioxide and water is referred to as the chemical oxygen demand (COD).73,74 COD test is used to determine the pollution level in a given location under examination. COD values are always greater than BOD5 values because several organic compounds can remain oxidized chemically but then not biologically.73,74 Thus, chemical oxygen demand (COD) is used in defining the level of pollution in water. Once the level of COD in the water exceeds 25 mg/L, it shows that there is higher concentration of pollutants. Furthermore, if the levels of COD exceed 50 mg/L, it is indicative that there is severe pollution which can be toxic for aquatic life. COD values were found to be highest at location 4 (41.78–32.49) mg/L during rainy and dry season. This indicates that organic pollution of water is more severe during rainy season than dry seasons. Thus, convincing the statement that organic pollution is worse at rainy season is more credible. All reported values in this investigation were above the maximum acceptable limit of 10mg/L for COD, as the trend demonstrates increasing chemical activity and may perhaps remain linked to areas prone to gas flaring as well as oil spill related activities impact. As COD is used to calculate the amount of oxygen required by organic and inorganic substances.73,74 For acidity, this ascends from presence of mild or strong acids as well as certain salts inorganic. In unpolluted surface and ground waters, the existence of dissolved carbon dioxide is commonly the dominant acidifying agent. Apart from a palatability problem in very acidic waters, there is no specific implication.69 The water acidity affects its corrosiveness as well as its speciation of other components. Thus, acidity values range from highest at location 3 (101.18–168.82) mg/L for both rainy season and dry season (Table 3&4). There is currently no maximum value set for acidity according to WHO/SON/NAFDAC standards of potability. Also, the existence of bicarbonates generated in soil reactions via which water infiltrates contributes to the alkalinity of the natural water body. It’s a measure of water's ability toward neutralizing acids as well as indicates its buffer capacity.69,9 Thus, bicarbonate and hydroxides may be responsible for alkalinity in natural waters as the eutrophication effects on water are influenced by alkalinity.69,9 Alkalinity was highest at location 4 & 8 (134.96-64.58) during the rainy and dry season and lowest at location 3 & 5 (18.17–23.66) during rainy and dry season correspondingly.
The high rate might remain attributed toward constant discharge of acidic and chemicalized substances through oil spillage and gas flaring which latter find their ways into the groundwater bodies and adjoining environment. Traditionally, for hardness, it is the measure of water's ability toward reacting with soap as well as characterizes water's ability toward binding soap to form scum or lather, which is a reaction that is chemically harmful toward the process of washing.9 Hardness of water is caused by groundwater interacting with rock structures. It is the total of the dissolved polyvalent metal ions concentrations, the most abundant of which remain Ca2+ and Mg2+. Calcium hardness, regardless of the salts involved, is referred to as calcium hardness. Similarly, magnesium hardness refers to hardness caused by magnesium. Because calcium as well as magnesium remain the only remarkable minerals that are known to induce hardness. The sources of the metallic ions remain often found in sedimentary rocks, the most prevalent of which remain limestone (CaCO3) as well as dolomite (CaMg(CO3)2). Total Hardness (TH) as calcium carbonate, is the sum of Ca2+ as well as Mg2+ in groundwater and signifies the existence of alkaline earths. Hardness can induce encrustation of water supply distribution systems. The concentration of TH levels varied from 39.17mg/L to 44.02mg/L [both rainy and dry season]. More than half of the sample’s groundwater in Ebocha-Obrikom area of Rivers State possess TH below 100mg/L. Thus, total hardness (TH) of the aquifers fluctuated on average from 39.17mg/L for rainy season to 44.02 mg/L for dry seasons, with the lowest confined groundwater (mean=35.02 at location 5) (35.78 at location 9), and highest confined groundwater (mean=42.22 mg/L at location 3) (51.66 at location 5). TH represents the total concentration of Ca2+ as well as Mg2+ in this system, which was mostly caused by mineral dissolution like carbonates as well as gypsum75 discovered that the increased groundwater hardness was caused by carbonate sources. The research results contradicted Disli (2017)'s finding that TH level varied “from 198.5 to 409.5 mg/L, with a mean of 289.1 in the Upper Tigris River Basin, Diyarbakır–Batman, Turkey”. Furthermore, in the crystalline basement complex rock of India, Adimalla et al. (2018a) recorded TH values ranging from 60 to 750 mg/L, with a mean of 202 mg/L, and approximately 18% of the samples falling into the moderately hard category, whereas77 obtained TH values ranging from 50.8 to 272 mg/L, with 60.6% of samples falling into the moderately hard category. Nonetheless, the highest permitted amount of TH aimed at drinking purposes is 500 mg/L, with a preferred limit of less than 100 mg/L (WHO, 2017).
The groundwater in the Ebocha-Obrikom oil and gas area was found to be 100% safe, with all samples falling within the maximum permitted 500 mg/L limit. Conversely,76 claims that subsurface waters remain often tougher than surface waters. While, Total Dissolved Solids (TDS) on the other hand, refers to the various minerals that remain existent in water in dissolved form and is a pointer of water salinity as well as signifies dissolved salts in water, such as large calcium, chlorides, bicarbonates, phosphates, sulfates, carbonates, silica, potassium, magnesium and sodium.78 TDS levels above a certain threshold impair the palatability of water as well as promote gastrointestinal discomfort in consumers. Consuming water of high TDS for an extended period of time can result in kidney stones. Consequently, it's a crucial factor to consider while assessing drinking water consistency as well as other water forms. It is also, an important metric for determining the appropriateness of irrigation as well as drinking water. WHO (2017) claims that groundwater taste with a TDS level of less than 600 mg/L is regarded good for aquatic lives, fisheries and residential water supply protection, but TDS levels greater than 1000 mg/L remain well-thought-out as unpalatable for the purposes of drinking? Thus, TDS levels beyond the WHO groundwater specified threshold may cause unpleasant taste as well as gastrointestinal complications. High TDS maybe derived from intensive or massive usage of agrochemical, dissolution of salts, ion exchange, organic materials, and sediment dissolution, aquifer percolation and allied substances emanating from oil related activities such as gas flaring as well as oil spillage.79 Thus, groundwater contamination in this wise could be due to the continuous contamination of groundwater by industrial pollutants as suggested. Although, all values were significantly below the acceptable limit, these total dissolved solids (TDS) show a very weak variability as seen by their low standard deviation (SD). Its concentration varies from 9.57mg/L to 12.34mg/L [both rainy and dry season]. The overall hydro chemical groundwater characteristics are regulated by major ions.9 Hence, the groundwater samples were desirable as well as allowed for purposes of drinking based on the TDS categorization of all collected groundwater samples. Thus, the difference in the concentration of TDS indicates a wide variation in the geochemical processes. Meanwhile, if the waters are to be utilized in fish ponds, WHO (2017) recommends a concentration of 1500mg/L for fisheries and aquatic life protection, and for household water supply. Because all values remained below the tolerable limit, they remain safe for drinking on TDS basis as supported by researches from Dami et al.,80 that waters within the limits attained here remain extremely palatable. Thus, all of the sample’s groundwater showed TDS levels that were lower than the WHO (2017) standard (250 mg/L) as well as the NAFDAC threshold levels (500 mg/L). Concentrations of TDS in Ebocha-Obrikom area remain below the optimal threshold in all locations, according to earlier research, Besides, TDS concentrations remained sufficient in quality for drinking in all areas. According to Adimalla81 & about 95% of the total samples remained below the ideal drinking threshold (TDS: 1000 mg/L), with the samples remaining appropriate for irrigation (TDS: 1000–3000 mg/L).
The mean values for total suspended solids (TSS), demonstrate that the greatest value in groundwater was witnessed at location 1&8 (39.80–34.76) mg/L for rainy and dry seasons. While, the least value of (34.00–29.56) mg/L at location 9 for rainy and dry seasons respectively. All of the values noted in this investigation were above the maximum allowable limit. Indicating that gas flaring and oil spillage releases persistent non-combustible chemicals and less dense volatile chemicals into the environment. In addition, it could be due to massive percolation of water through the water table as well as the discharge of huge amounts of pollutants straight into groundwater bodies or onto terrestrial regions where they leach into bodies of ground water. In terms of salinity, all groundwater comprises salts solution; and documented salt contents extending from less than 25mg/L in a quartzite spring to above 300,000mg/L in brines.82 The kind as well as salts concentration depends on the milieu, mobility as well as groundwater source. Because of the larger exposure toward soluble elements in geologic strata, groundwater often has a larger concentration of dissolved components than surface water. Groundwater soluble salts are generally formed by dissolving rock minerals? Bicarbonate, which is typically the predominant groundwater anion, is produced from the released of carbon dioxide in the soil by organic breakdown. Salinity differs with aquifer material specific surface area, minerals solubility as well as contact duration; values tend to remain maximum where groundwater circulation is minimum; hence salinity mostly increase with depth. Thus, salinity values range from highest at location 7 (25.71) mg/L for rainy season to location 3 (16.00) mg/L (Table 3&4). The maximum value is set at 600 mg/L according to WHO/SON/NAFDAC standards of potability. All of the readings obtained in this study were less than the maximum allowable limit of 600 mg/l for drinking water. Chloride can be present in a variety of chemical and non-chemical components in the body. It is an essential component of the salt found in many foods and used in cooking. It is also a component of the digestive (stomach) juices. Sodium chloride can be found in table salt or sea salt. Even in small children, too much chloride from salted meals can raise blood pressure56,9 can promote fluid buildup in persons suffering from congestive heart failure, cirrhosis, or kidney disease (www.health.nytimes.com). Even though excessive use of drinking water containing sodium chloride at concentrations greater than 2.5 g/litre has been linked to hypertension, this effect is thought to be connected to sodium ion concentration.
Thus, chloride is an inert as well as mobile compound, whose natural amount is determined by sea distance, geographical location as well as precipitation amount, but also by the regional impact of groundwater resulting from saline water inputs. A number of studies alleged that Cl excess in groundwater is an indicator pollution index and has a harmful influence on human health.83,9,75 Though, chloride is also one of the prominent anion in Rivers State oil and gas producing area of Ebocha-Obrokom, ranging from (28.33–32.16)(25.26–31.27) mg/L for both rainy and dry season with a mean of (30.60–28.21) mg/L (Table 3&4). When compared to the acceptable limit of 200 mg/L, all of the samples had lower concentrations, which might be attributable to higher neutralization reactions by dissolved alkaline hydroxyl-containing substances. It has been noted that while water with low chloride ions is not dangerous, chloride ions at large concentrations can kill floras when used for horticultural or agricultural applications. It may also be to blame for the unpleasant taste of water consumed (WHO, 2004). While samples at location (3 & 5) for rainy and dry season were high in the Ebocha - Obrikom region. These samples were collected at the center of the research area. Elevated levels of chloride may perhaps remain linked to domestic waste effluents, septic tanks leakage, as well as chloride bearing rocks dissolution.84,85 Thus, the numerous natural as well as chlorides anthropogenic sources, concentrations of ambient background vary greatly. In spite of the fact that no health dangers have been established, residents of Ebocha-Obrikom areas remain hesitant toward drinking water due to texture as well as taste issues. Hence, high Cl− groundwater concentrations remain seen as a symptom of pollution from a number of sources, and they impart a salty flavor to the water.85,9 In addition, Chloride concentration in drinking water above 200 mg/l have been linked to heart disease, asthma as well as possibly cancer. For fluorine (F−), it is widely distributed in the environment. It is usually safe to drink water within the limits of 0.5–1.5 mg/l according to the suggested guidelines. Fluoride becomes harmful to health at quantities above/below this recommendation, and is denoted as a double-edged sword. Water consumers remain prone to dental carries at lower concentration, while at larger concentrations, it can induce skeletal fluorosis, debilitating fluorosis, dental fluorosis, as well as kidney damage.9 Excess consumption of fluoride has also been associated to infertility, abortion, fertility, as well as hypertension.
Water ingestion as well as skin absorption remain the primary sources of trace elements intake in the milieu.9 While fluorine remains the 13th most prevalent element in the earth’s crust, it is essential to human life. Elemental fluorine almost never occurs in nature, but fluoride is a shared element that is broadly dispersed in the earth’s crust as well as appears in fluoride form in a number of fluoride rich minerals, like fluoroapatite (Ca5(PO4)3F), fluorite (CaF2), fluorspar, apatite, amphiboles, hornblende, villiaumite (NaF), micas, cryolite (Na3AlF6), as well as topaz (Al2(SiO4)F2). Heavy groundwater fluoride concentrations are a pervasive problem around the globe; particularly in the global south, where individuals remain disproportionately impacted by fluorosis due to high reliance on groundwater with fluoride contents above the normal threshold. Also, excessive level of fluoride in drinking water might result in a decrease in total erythrocyte, haematocrit value, haemoglobin percentage, as well as protein content. Fluoride (F−) is a common groundwater contaminant in loess environments. F– occurs naturally in groundwater as a result of fluoride-containing minerals dissolution. In trace amounts, fluoride is advantageous to the human health as well as can minimize dental caries risk even though encouraging strong bones formation.87 According to the WHO (2017), the maximum permissible F− limit in drinking water is 1.5mg/L. Usually, F− in groundwater in the research area is mostly caused by the fluoride-containing rocks dissolution as well as high-fluorine coal.88 In this study, [F−] in groundwater either fall below or within the WHO/SON/NAFDAC limit. The lowest as well as highest values (0.00 and 0.85mg/L) for rainy season (0.00 and 1.02mg/L) for dry season were observed in Ebocha-Obrikom area of Rivers State. Aside from the increased hydrodynamics during gas flaring production, which accelerates F− release from fluoride-containing minerals as well as high-fluorine coals, hydraulic connectivity as well as leakages during gas flaring production might result water mixing from diverse aquifers, affecting concentrations of F− in location 4, 5, 6, 7, and 8 [both rainy and dry season]. Chronic exposure toward fluoride raised concentrations in drinking water exceed World Health Organisation recommendation limit of 1.5 mg/L frequently leads to endemic diseases like skeletal as well as dental fluorosis (WHO, 2011). Aluminium had highest recorded value of (0.03) (0.02)mg/L at location 7 during the rainy and dry season. No values recorded at location 1, 2 & 9 (for rainy season) and location 1, 2, 3 & 9 (for dry season). For these locations, it shows that aluminium does not pose any health and environmental threat to consumption of ground water sources. However, its presence in other locations could be attributed to gas flaring and this of course calls for serious concern. Sodium [Na+] is abundant in rocks and soils. It is always present in natural water. It is used medicinally as a laxative.57 Sodium [Na+] varied from 14.33mg/L in rainy season to 11.39 mg/L in dry season, which was below the maximum allowable limit of 200 mg/L for drinking uses. Still, the highest [Na+], 16.39 mg/L was observed in location 7 of the unconfined groundwater, while the lowest mean [Na+], 12.22mg/L, was observed in location 3 of the unconfined groundwater, suggesting that Ebocha-Obrikom oil and gas area of groundwater is affected by more complex factors. Overall, Na+ had the highest concentration in location 2, 4, 5, 6, 7 & 8.
These ions in groundwater are largely regulated by weathering and water–rock interactions. Also, it can be attributed to longer residence time, mineral composition, and greater water–rock interaction intensity in the aquifer. Thus, the excess of Na+ also indirectly indicates the process of ion exchange in water formation.89 In the Ebocha-Obrikom oil and gas area, principal lithology is occupied by crystalline rocks. Therefore, weathering of these rock forming minerals might likely be the chief source for elevated Na+ concentration in Ebocha-Obrikom oil and gas area. For potassium (K+), it usually exists at low concentrations in groundwater because of weak mobility.90 In this study, [K+] ranged from 2.93 mg/L for rainy season to 2.82 mg/L for dry season in the aquifer. The highest [K+] (3.29 mg/L) was observed in location 8 of the shallow confined groundwater, while the control groundwater had the lowest mean [K+], 2.42mg/L at location 9. Thus, K+ is present in a low amount ranging from 2.42 to 3.29mg/L. The concentrations of Ca2+, Mg2+, and Na+ range between (50.31 - 59.98)(40-37 - 50.73), (129.26 -146.67)(149.44 - 184.68), and (12.22 - 16.39)(9.22 - 13.33)mg/L, respectively. Mg+ possesses the highest SD value, indicating a very high spatial variability. Thus, He and Wu91 reported that the K+ in groundwater is one of the very necessary trace elements to maintain human health. K+ occurs naturally in drinking water at quantities far below those considered harmful to human health and is the most important nutrient for humans and too much of it might cause constipation (WHO, 2017). However, it is shown that the high level of Sodium (Na+) and Potassium (K+) in drinking water (beyond the standard limit) may cause hypertension, high blood pressure, hyperkalemia, and often cause a heart attack. For calcium, this element is the most important and abundant in human body and adequate intake is essential for normal growth and health. There is some evidence to show that the incidence of heart disease is reduced in areas served by public water supply with a high degree of hardness, the primary constituent of which is calcium, so that the presence of the element in a water supply is beneficial to health (USEPA, 2015). Hence, calcium is one of the dominant cations in the Ebocha-Obrikom oil and gas area of Rivers State groundwater, ranging from (50.31-59.98) (40.37-50.73) mg/L with a mean of (54.80–45.37) mg/L. According to WHO (2017), the most desirable concentration of Ca2+ in groundwater for drinking uses is 75 mg/L and the maximum allowable limit is 200 mg/L. However, the present study results of Ca2+ indicated that groundwater samples were below the most desirable limit of 75 mg/L, which can be used for drinking purposes.
While, Ca and Mg are present as simple ions Ca2+ and Mg2+ with the Ca levels varying from tens to hundreds of mg/L and the Mg concentrations varying from units of tens of mg/L. While, calcium and magnesium are essential for the human body (WHO, 2011). They contribute to the formation and solidification of bones and teeth and play a role in the decrease of neuromuscular excitability, myocardial system, heart, and muscle contractility, intracellular information, transmission, and blood contractility. They also play a major role in the metabolism of almost all cells of the body and interacts with many nutrients. However, inadequate, or excess intake of either nutrient can result in adverse health consequences (WHO, 2011). According to WHO (2011) “Inadequate intakes of calcium have been associated with increased risks of osteoporosis, nephrolithiasis (kidney stones), colorectal cancer, hypertension and stroke, coronary artery disease, insulin resistance and obesity. Most of these disorders have treatments, but not cures. Owing to a lack of compelling evidence for the role of calcium as a contributory element in relation to these diseases, estimates of calcium requirement have been made based on bone health outcomes, with the goal of optimizing bone mineral density. To a great extent, individuals are protected from excess intakes of calcium by a tightly regulated intestinal absorption and elimination mechanism through the action of 1,25- dihydroxy vitamin D, the hormonally active form of vitamin D. When calcium is absorbed more than need, the excess is excreted by the kidney in healthy people who do not have renal impairment” (WHO, 2011). In the Ebocha-Obrikom oil and gas producing area of River State groundwater, concentration of magnesium (Mg2+) ranged between (129.26–146.67) (149.44–184.68) mg/L both rainy and dry season, with an average mean of (135.18–174.61) mg/L (Table 3&4). The major source of Mg2+ in the groundwater was magnesium bearing minerals in the host rocks and also animal, domestic, and industrial wastes. However, all collected groundwater samples were above the maximum allowable limit of 150 mg/L (WHO, 2017). Thus, the high concentration of Mg2+ in groundwater may be because of the occurrence of exchangeable Na+ in the soil. High magnesium hazard (MH) values reveal damage to the soil structure due to a subsequent increase in soil alkalinity. Although, magnesium is significantly less abundant than calcium in rocks and in most natural waters. In addition, magnesium concentrations are much lower in the water than calcium. They are generally less than 50 mg/L, although values higher or equal to 100 mg/L are stored particularly in cold climates.92 Magnesium being the fourth most abundant cation in the body and the second most abundant cation in intracellular fluid (WHO, 2017).
In the cardiovascular system, magnesium is the candidate element. It plays an important role as a cofactor and activator of more than 300 enzymatic reactions including glycolysis, ATP metabolism, transport of elements such as Na, K and Ca through membranes, synthesis of proteins and nucleic acids, neuromuscular excitability and muscle contraction.93 That can have hand in various mechanism where the main is the calcium antagonist effect which can be direct or indirect.93 Low magnesium levels are associated with endothelial dysfunction, increased vascular reactions, elevated circulating levels of C-reactive protein (a proinflammatory marker that is a risk factor for coronary heart disease) and decreased insulin sensitivity. Low magnesium status has been implicated in hypertension, coronary heart disease, type 2 diabetes mellitus and metabolic syndrome. Magnesium deficiency has been implicated in the pathogenesis of hypertension, with some epidemiological and experimental studies demonstrating a negative correlation between blood pressure and serum magnesium levels. However, data from clinical studies have been less convincing (WHO, 2011). Thus, Ca2+ and Mg2+ are very essential for humans, but excessive intake of them may cause negative human health impact. While, Iron is a lustrous, ductile, malleable, silver-gray metal. Its presence in human tissue for extended periods may cause conjunctivitis, choroiditis and retinitis. A common problem for human is iron deficiency, which may lead to anemia. A man needs an average daily intake of 7mg of iron and a woman 11mg. Presence of Iron in water can lead to change of colour of groundwater. In addition, Iron is a highly redox-sensitive element that has low background concentrations in unconfined aquifers, where oxygen is present, but background concentrations of iron can be increased significantly across redox boundaries. It is evident that trace metal can be toxic to human health if they are consumed in excess and accumulated in human bodies.94 In this study, the concentration of Fe ranges from (1.21–5.16)(0.95–4.42) mg/L both rainy and dry season, and four (4) samples in rainy seasons and two (2) samples in dry season have the Fe concentration higher than the permissible limit for drinking purpose. Thus, indicating fast and pronounced reductive dissolution of iron species in anoxic groundwater. It is well-known that water-quality thresholds may be frequently breached for iron, which occur in groundwater by natural processes, such as the geochemical conditions existing in the aquifer or due to the specific geology of the area. Thus, high concentrations of iron could result in hemochromatosis which is characterized by tiredness, pains in the joints and abdomen. Zinc is an important mineral apparent by the human today as being excellent biologic and human health significance, mainly concerning prenatal and perinatal growth. Zinc deficiency effects around two billion public in the rising global and is related with several illnesses. In children its sources development delay late sexual development, infection vulnerability, and diarrhea. While, the highest value for zinc was observed at location 3 (0.77)mg/L for rainy season and location 4 (1.01)mg/L for dry season. It was noticed that the maximum permissible limit of 3.00mg/L for zinc was not exceeded by any of the locations.
Zinc at these limit does not pose serious health and environmental effects though significant values were noticed at locations stated above between the seasons. Thus, zinc could be deposited in those locations due to oil related activities, especially during dry season. Studies have documented that the harmful effects of zinc on people include nausea, lack of moisture, tiredness, pains in the abdomen, lack of coordination of the muscles, and kidney failure. Although a normal dose of zinc is essential to prevent zinc deficiency, protracted dosages of zinc could trigger the development of deformed blood cells and the impairment of the pancreas. In addition, zinc insufficiency is generally due to unsatisfactory dietary consumption, but can be correlated with malabsorption, Acrodermatitis enteropathica, liver damage, renal damage, sickle cell damage, diabetes, malignancy, and other chronic diseases assemblies at hazard for zinc deficiency contain the elderly, children in rising nations, and individuals with renal deficiency. Signs of mild zinc insufficiency are varied. Thus, medical consequences contain depressed development, diarrhea, weakness and late sexual development, alopecia, eye and skin abrasions, decreased appetite, changed perception, decreased host protection possessions, defects in carbohydrate utilization, and reproductive spermatogenesis. Manganese is a redox-sensitive element, it is influenced by the same processes that determine the reductive dissolution of Fe oxy-hydroxides and as desorption from their surfaces. The maximums value of Mn and Zn are (0.04–0.08) and (0.77–1.01) mg/L for rainy and dry season respectively, and all groundwater samples are suitable for drinking in terms of the Zn concentration, but two (2) samples are not suitable for drinking (location 2&3–4&5) because of high Mn concentration in groundwater. While, manganese is an essential nutrient, manganese is neurotoxic at high levels of exposure and evidence suggests that infants could be uniquely vulnerable to its effects. Manganese exposures in drinking water have been associated with neurodevelopmental outcomes that include reduced IQ or poorer memory, inattention, hyperactivity, impulsivity and motor function in children.95 Thus, water that exceed the state’s reference dose (RfD) for manganese, which is the highest daily intake that is estimated is likely to cause harmful effects over a lifetime of exposure. This finding should be seen as a wake-up call. As this is not just an Ebocha-Obrikom issue. High water manganese levels are likely prevalent in many parts of the core Niger Delta Region. The study showed that groundwater in Ebocha-Obrikom area of Rivers State contain widely varying amounts of manganese. This study is significant because it calls attention not only to high levels of manganese in groundwater, but also the important fact that many small communities in the Niger Delta region could have elevated manganese in their ground water systems, Thus, residents of the Ebocha-Obrikon community should be aware of the manganese levels in their ground water particularly if they have infants or young children. Cadmium (Cd) are known to increase the risks of lung cancer and renal carcinoma. The highest value for cadmium was observed at location 2, 7 &8 (0.02)mg/L during the rainy season and location 4 &7 (0.06)mg/L during the dry season. All values recorded in this study area were above the maximum permissible limit of 0.003mg/l for WHO/SON/NAFDAC.
Thus, Cadmium (Cd) is known to cause damage to the kidney, bones in both young and old, also responsible for bronchitis, anaemia. Lead is classified as a prevalent toxic metal and a major environmental health hazard, when it enters into the chain through drinking water and crop irrigation. It can accumulate in bone, muscle, liver, kidney and brain. Excessive lead causes problems in the synthesis of hemoglobin, kidney disease, mental retardation, anemia and acute or chronic damage to the nervous system. Pb2+ is very toxic to human beings when present in high amounts. Since Pb2+ is not biodegradable, once soil has become contaminated, it remains a long-term source of Pb2+ exposure. The primary cause of lead’s toxicity is its interference with a variety of enzymes since it binds to sulfhydryl groups found in many enzymes. Lead also interferes in the activity of an essential enzyme called delta-aminolevulinic acid dehydrates, or ALAD and ferrochelatase which are important in the biosynthesis of heme, the cofactor found in hemoglobin. Approximately, contact to lead is growing above period. Extreme level of lead absorptions in the human body can cause death or perpetual harm to the brain, central nervous system and kidneys. Lead being one of the most poisonous heavy metals because of its impact on the kidneys and central nervous system. The highest value for lead was observed at location 7 (0.14)mg/L during the rainy season and location 3 (0.03)mg/L during the dry season. All values recorded in this study area were either within or above the maximum permissible limit of 0.01mg/l for WHO/SON/NAFDAC. Thus, could be said to be of environmental and health concern. As long-term exposure to lead (Pb) can be harmful to the circulatory and central nervous systems. Thus, lead is a hazardous component; it is injurious even in minor quantities. Lead component comes in the human body majorly found in water and food. It can be gasped in powder form of lead in paints, or excess gases from leaded petroleum products. It is originated in minor quantities in several water bodies and food, particularly fish, which remain seriously focus to industrialized toxic waste. Lead reduces enzymes nonfunctional by compulsory to their sulfhydryl group’s additional contribution to a damage in oxidative balance. The capability of lead to permit above the barrier blood and brain is mostly due to its capability to extra for calcium ions. Major toxicity of lead causing the brain prefrontal hippocampus, cerebellum and cerebral cortex can lead to a variability of neurological disorder, such as brain injury, psychological delay, behavior difficulties, nerve injury, and probably Alzheimer’s disease, Parkinson’s disease and schizophrenia. Copper is a reddish metal with a face-centered cubic crystalline structure. It can be found in many kinds of food, in drinking water and in air. Long-term exposure to copper can cause irritation of the nose, mouth and eyes and it causes headaches, stomachaches, dizziness, vomiting and diarrhea. According to the Agency for Toxic Substances and Disease Registry (2015), intentionally high uptakes of copper may cause liver and kidney damage even death. Thus, copper is a ductile metal with very high thermal and electrical conductivity.
Copper occurs in nature in its metallic form and in ores and minerals. The metal and its alloys have been used for thousands of years. Copper is an essential dietary element for all living organisms in trace amounts, because it plays a major role as a key constituent of the respiratory enzyme complex cytochrome oxidase. Copper had its highest of (0.05)mg/L at location 6 for rainy season and (2.81)mg/L at locations 4 during the dry season. All values were below the maximum permissible limit of copper. However, studies have reported that high values of copper could lead to the development of chronic anemia. Contamination of drinking water by copper could be by either directly polluting water sources or through rusting of copper pipes and materials. Thus, copper being one of the most common pollutants found in industrial effluents, its extreme consumption of copper leads to gastrointestinal problems, kidney damage, anemia and lung cancer. Copper is lethal for human in the range from 4 to 400 mg/kg of body weight. Lower doses of copper ions can cause symptoms typical of food poisoning (headache, nausea, vomiting, diarrhea). In humans, the liver is the primary organ of copper-induced toxicity. Finally, in humans, copper poisoning causes Wilson's disease. Chromium is a naturally occurring metal that exists on the geological, with corrosion states ranging from chromium. Chromium enters many environmental media (air, soil and water) through a wide range of natural as well as anthropogenic sources with the majority of discharge originating from industrial establishment. Metal dispensing, welding stainless steel, chromate manufacture, tannery services and ferrochrome as well as chrome pigment manufacture are among the industries having a significant participation in chromium discharge. Thus, chromium is a highly toxic element due to its ability to penetrate cell membranes and at high exposure level can cause liver damage as well as kidney, skin ulceration as well as impact the central nervous system. In addition, chromium (III) and chromium (VI) have very different toxicity characteristics. Chromium is essential for human nutrition and is considered non-toxic. Levels more than 0.05 mg/L of chromium (VI) in drinking water can result in convulsions, diarrhoea, abdominal pain, vomiting, indigestion, as well as damage to liver and kidney. Compounds containing hexavalent chromium are genotoxic carcinogens. Inhaling hexavalent chromium compounds on a regular basis raises lung cancer risk. chromium (VI) ingestion can potentially induce stomach and intestinal discomfort or ulcers. In this study, chromium had its highest of (1.29)mg/L at location 5 for rainy season and (2.81)mg/L at locations 4 throughout the dry season.
All values remained above the maximum permissible chromium level. Although, chromium does not pose any known serious environmental and public health threat, but its current concentration must be continuously monitored since it may perhaps be attributable to gas flaring. The contamination of ground water by chromium could be as a result of exposure to wastes from oil and gas processing facilities which are indiscriminately disposed into open dumps. Chromium in its hexavalent form has been associated with the injurious effects of chromium to mankind. Harmful effects of chromium include liver necrosis and membrane ulcers. Hence chromium (Cr) is an important trace component involved in basal metabolism, excess of it in the body can be harmful, in part because Cr is a documented human carcinogen as well as anaphylactogen. Chromium exposure in the environment involves compound mixtures identified to cause multi organ poisonousness like allergy, asthma, kidney damage and, in severe cases, cancer of the respiratory tract in humans. Impatience as well as small intestine ulceration and anemia, abdominal, impairment to the male reproductive system as well as sperm destruction are the most serious health problems reported in humans after consuming chromium combinations. Almost everyone is extremely chromium sensitive, allergic responses such as significant swelling as well as skin redness have been observed. People and animals exposed to chromium in water experienced an increase in abdominal cancers. Excessive chromium complexes consumption by humans resulted in obvious hepatic, respiratory, renal, gastrointestinal, cardiovascular, hematological, and neurological effects as part of the sequelae significant to death or in patients who continued as per medical therapy. Despite the fact that evidence of chromium carcinogenicity in humans and other living things appears to be difficult to come by. The sulphate (SO42−) concentrations were within permissible limit in the groundwater samples. As shown in Table 3&4, SO42− ranged from 0.87mg/L in rainy season to 1.01mg/L in dry season. The highest concentrations of (SO42−) were observed in location 4 (0.99mg/L) and the lowest was observed at location 7 (0.84mg/L), these groundwater concentrations of these anions, particularly SO42− are influenced via hydrogeological as well as climatic conditions and anthropogenic influences. Evaporation (a climatic component) is very severe in the area. Furthermore, hydrogeological (water–rock interactions and weathering) as well as human caused factors (industrial activities and agricultural) led to the rise of significant anion concentrations. The general groundwater properties in a specific area remain determine via major ions. Whereas unconfined as well as semi-confined aquifers within the water bodies could be related to remarkably lower levels. The highest value of sulphate at location 4 & 2 could remain found in water body (Table 3&4), indicating a significant sulphate sensitivity toward changes in geochemical characteristics within the aquifer system.
Also, it could be stated that gas flaring must have increased the concentration of sulphate during the dry season as against the low levels during the rainy season. In addition, it may perhaps be related to agricultural pollution from fertilizers that leached underground and mixed through ground water. It may be said that sulphate is particularly unstable in the atmosphere, where it is transformed into forms ideal for its long-term presence in groundwater’s. High sulphate concentrations are widely recognized to be caused by minerals dissolution that govern its water natural abundance or by changes in land use. Sulphate is particularly mentioned in the EU Groundwater Directive (2006/118/EC) as an indicator of saline intrusion caused by human activities. In unconfined aquifers inside a water body, an increase in sulphate content is visible due to geogenic processes and human effect, which can be connected with strong human impact from domestic diffuse pressure or water abstraction in the body. Thus, excessive sulfate use can have a laxative impact. For ammonia, Nutrient salts (nitrite, nitrate and ammonia,) are vital to the metabolism and growth of aquatic life, and when their concentrations rise, the biological balance shifts. Human activity has caused a significant increase in the amount of nutrients and salts in aquatic ecosystems, causing an issue with water quality. Extensive use of mineral fertilizers in some areas has resulted in atmospheric pollution, greenhouse gas emissions (e.g., N2O, which is significant for climate regulation), eutrophication of water, as well as human health risks all of which have a negative impact on regulating services of soil, air, as well as water quality. Excess nitrogen inputs can also cause eutrophication, which is the increased creation of organic matter as well as the potential formation of areas with low oxygen dissolved concentration (hypoxia). Hypoxia could result in the mortality of benthic species, fish kills, diminished growth as well as life cycles disruption. Increased turbidity, loss of submerged aquatic vegetation as well as food web changes are all signs of eutrophication. Thus, ammonia (NH3-) values range from highest at location 6 (2.80) mg/L for rainy season to location 3 (4.39) mg/L (Table 3&4). The maximum value is set at 3.0 mg/L according to WHO/SON/NAFDAC standards of potability. Phosphorus is abundant in nature, appearing in microorganisms, plants, animal excrement, and so on. The importance of phosphorus is mostly related to the phenomenon of lakes eutrophication as well as to a lesser extent, rivers eutrophication. Phosphorus entering such bodies of water, combined with nitrogen as nitrate, promote algae growth as well as other plants resulting in blooms, littoral slimes, large diurnal dissolved oxygen disparity, as well as other problems. Phosphorus can be found in polyphosphate, orthophosphate, organic phosphate as well as other compounds (USEPA, 2015). Levels of phosphate in drinking water that are too high may induce digestive difficulties in animals and humans. Thus, phosphate had its highest value at location (2 & 3) (0.38) mg/L during the rainy season and location 8 (0.55) mg/L during the dry season. All values documented in this investigation exceeded the maximum allowable limit specified by SON/NAFDAC/ WHO.
This could be because farmers in the research study area typically nourished the farms with fertilizers of which phosphate fertilizer is one. Also, the high phosphate amount could be attributed to oil and gas related activities and contaminated rains as a results of gas flaring, falling and being retained within ground water within the study area. Also, for Nitrogen, it is an important component of the biogeochemical cycle; it can remain seen as numerous gases inside organic matter as well as dissolved organisms. The state of oxidation of nitrogen naturally varies from +5 in NO3 − to − 3 in NH4+. It is propelled by the reduction series described below:
NO3 −→NO2 −→N2(gas)→NH4+
Nitrate-contaminated groundwater is now a threat in all nations. The uncontrolled fertilizer use as well as massive waste creation, with consequent nitrogen leakage from soil, have raised severe concerns about nitrogen future in aquifers. Thus, an increased concentration of nitrate in drinking water induces a potentially deadly condition in which oxygen delivery in the bloodstream is hindered (methemoglobinemia). While, NO3− is commonly used as an indication of agricultural nonpoint source pollution,97 it can also be harmful to human health.96 The allowable NO3− limit in drinking water is 50 mg/L (WHO, 2017). NO3− level in groundwater ranged from 1.79mg/L to 3.30 [both rainy and dry season]. Nitrite groundwater pollution is thought to have started in both industrial and agriculture field. High [NO3 −] levels in drinking water may cause human health problems, including the emergence of blue baby or methemoglobinemia patients. It is unusual to find NO3− in deep groundwater. As a result, if NO3− is found in deep groundwater, it is most probable due to the significant hydraulic connection between the borehole and well aquifers. Other variables, however, influence [NO3−] in deep aquifers. Nitrogen emitted by gas flaring may perhaps remain a source of NO3−. Burning of gas flaring could also be a possible source. While, Nitrates (NO2−) are one of the qualitative parameters of groundwater as well as their enrichment has human health repercussions, it necessitates exact periodic extent. Nitrate is regularly observed in high quantities in unconfined alluvial aquifers, and its origin can be connected in some circumstances to the presence of higher chloride as well as sulphate concentration, especially in urban areas. While, nitrate (NO2−) is among the most common groundwater pollutants worldwide. Its high concentration primarily causes severe health problems, like gastric cancer, methemoglobinemia, birth malformations, goiter as well as hypertension. In loess areas, NO2− is a prevalent pollutant in groundwater. Nevertheless, excessive NO2− concentration in groundwater are thought to be caused by anthropogenic activities like fertilizer application in agriculture as well as sewage wastes leaching or poultry manure. NO2− levels in this study vary from (1.61 – 2.33)(2.31–4.57) mg/ L, indicating that groundwater samples at location 2, 3, 6 & 9 for rainy season are suitable for drinking according to the WHO/SON/NAFDAC drinking water guidelines (WHO, 2017). Thus, availability of nitrogen fixing bacteria on leguminous root nodules that penetrate atmospheric nitrogen into the soil could account for the very low levels of nitrates. However, the concentrations of NO2− during the dry season in the studied groundwater samples are high, with the highest value being 4.57 mg/L. This could be due to the twin factor of oil spillage and gas flaring residual effects. Although NO2− concentration is below the permissible limit of NO2− for drinking purpose (WHO, 2017). As mentioned earlier, oil and gas related activities as well as agriculture are the primary occupations and sources of income in Ebocha-Obrikom oil and gas producing area of River State; therefore, high concentration of nitrate in groundwater is mainly attributed to sewage leaks, household discharges not connected to sewage systems and activities of agriculture, where the usage of N-fertilizer (organic manure and synthetic nitrogenous fertilizers) is communal. This result was contrary to the study conducted in Nuevo-Leon, north-eastern Mexico, where 82 percent of the sampling sites remained affected via sources of anthropogenic in an agricultural area.98 Several studies have identified a strong correlation between agriculture as well as groundwater nitrate concentrations. Thus, in the 21st century, indigenous Ebocha-Obrikom drinking water supply would undoubtedly encounter problems from lead pipes, algal blooms, nutrient contamination, as well as other concerns. Conclusively, intensive agricultural operations like fertilizers application as well as other human actions like household sewage discharge might remain substantial contributors to groundwater nitrate pollution. As the land as well as climate conditions in the loess zones remain poor, and a substantial amount of fertilizer has been utilized to boost agricultural productivity, nitrate pollution is also highly widespread.99,100 For Nickel, it is typically found in the tissues of human as well as in high exposure scenarios, these levels can skyrocket. In the general population, nickel intake contributions via drinking water remain typically less remarkable than dietary consumption with absorption being the most major exposure mode. Nickel intake is determined by its physicochemical technique, with water-soluble techniques (nitrate, sulphate, chloride) providing additional readily consumed nickel. Thus, the values for nickel was higher at location 4 (1.00)mg/L for rainy season and location 3 (1.40)mg/L for dry season respectively. The values were higher than the WHO/SON/NAFDAC tolerable limits of 0.02 mg/L.
The nickel values differed remarkably. Even though nickel has been identified as a vital trace metal, it could also be highly poisonous at higher doses. Hair loss, lung fibrosis, allergies of the skin, eczema, and various degrees of kidney and heart poisoning have been associated with humans exposed to high concentrations. Nickel also has the propensity of replacing iron and zinc in the body, thus interfering in the normal biochemistry. Though distribution differences occur as a result of the exposure method, the solubility of the nickel element as well as the time following exposure, the lungs and upper respiratory tract for inhalation exposure and the kidney for oral exposure are the major target organs for nickel-induced general toxicity. The immune system, cardiovascular system as well as blood are further object organs. Exposure to severely nickel-polluted water is likely to result in a variety of clinical effects in human. Skin allergies, respiratory cancer tract, lung fibrosis as well as iatrogenic nickel poisoning are among them. It has remained linked that nickel exposure causes hematological consequences in both animals as well as humans. Even though no reproductive consequences have remained reported with humans’ exposure to nickel. In addition, the geographic distributions of TPH are depicted in Table 3 & 4, respectively. The research areas of location 1, 2, 3, 4 & 6 have higher TPH concentrations, while location 5, 7 & 8 have lower TPH concentration and in location 9 TPH was not detected for rainy season. The content of TPH in groundwater, on the other hand indicated that locations 2, 3, 4, & 7 had higher concentration above WHO/SON/NAFDAC standards, while location 1, 5, 6 & 8 had concentration below permissible standards. But location 9 did not show any presence of TPH for dry season. The findings revealed that five (5) locations in the rainy season and four (4) locations in the dry season did not fulfill the WHO/SON/NAFDAC criteria. Accordingly, the result demonstrates that TPH concentrations in drinking water remain significantly greater, implying that water quality may have an unfavorable effect on fish survival, eggs and production of larvae as well as ecosystem maturation. Because of the relatively high tidal velocities, the pollution is spread out over a vast area. The river turbulence, on the other hand, meant that the spill was broken down into smaller, less harmful pieces. There is also concern about the lengthy period required for total biodegradation of the heavier components, which contain very hazardous aromatic compounds with low boiling points. The high saturated boiling point as well as aromatic hydrocarbons possess the potential to interfere with aquatic creatures’ responses toward chemical stimuli, like sex desire with equally severe repercussions. The utmost harmful pollution aspect, and definitely all pollutions, is the toxicant amplification problem, because several of the crude oil components remain chemically stable as well as not easily metabolized or eliminated once taken, making them available in the food chain at all times. The high TPH values in those sites are a cautionary sign that everything is not well, since some water company and vendors use ground water for production as well as sell it in places nearby or as far away as Yenagoa and Imo. Aside from its deadly effects, oil can induce death via producing narcosis, which causes animals to get detached from substrate oil coating can induce death through asphyxiation in other creatures. Oil coatings on the water surface in damaged areas impede light transmission and thus photosynthetic primary production. In the interim, the physical as well as chemical consequence untoward effects as well as oil effects remain long-lasting. As a result, we must not forget that the general pollution effect of this sort and magnitude occurrence on water bodies as well as ecosystem is significantly more problematic to anticipate. As a result, total recovery may perhaps take close to 20 years. In summary, trace metals cause respiratory irritation, kidney failure, neurological impairments, immunosuppression, anemia, gastrointestinal as well as cancer of liver, skeletal system abnormalities, liver inflammation, cardiovascular diseases following chronic exposure. Diagrammatically, the main contaminants effects on human health (see Figure 7 below) is represented thus.101–130
Pollution is a worldwide issue with no boundaries. Contaminants can be found on every continent, even in the most distant locations, and they can easily be moved from one country to the next. Thus, since ancient times, human activities have produced thousands of different manmade chemical compounds and naturally occurring components with potential toxicity into the environment. These contaminants have a long residence duration in the environment, ranging from hundreds to thousands of years, and are found all over the world. Hence, groundwater pollution has become as one of the most serious threats to human health, but its consequences extend far beyond the dimensions as well as contaminants, as it can have irreversible consequences for human as well as ecosystem health, result in severe economic losses as well as social inequities and jeopardize the 2030 Agenda for Sustainable Development achievement. Hence, preferred groundwater is the water resource supply around the world, particularly in the global south, where water is scarce and of poor water quality. the available assumption as well as sustainable confined groundwater is hydrochemistry, which must remain concerned preferentially. The Ebocha-Obrikom oil and gas producing area of River State, was chosen as the study area in order to gain awareness into the hydrochemistry as well as perspective of groundwater health and to make available decision-useful information as well as assist in taken action to solve the urgent threats facing societies across the Niger Delta, while fostering understanding as well as collaboration through a diverse stakeholder’s range of actionable solutions. The following highlights are offered after the broad research findings:
I thank Prof. Mynepalli K.C. Sridhar, Prof. Oyeyemi Abisoye Sunday, Prof. Kalada G. Mcfubara, Prof. Bassey E. Akpan, Prof. Innocent M. Aprioku as well as all anonymous reviewers, for feedback and discussions that helped to substantially improve this manuscript.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The water sampling and analysis was supported by AGIP Research Department.
The data that support the findings of this study are summarized in the various tables and are available without undue reservation from the corresponding author while the data are submitted to the Figshare web portal:
https://doi.org/10.6084/m9.figshare.14273234.v1.
The Supplementary Material for this article can be found online at: Raimi, Morufu (2021): Water Quality Parameters in Gas Flaring Area of Ebocha-Obrikom of Rivers State, Nigeria. Figshare. Dataset.
https://doi.org/10.6084/m9.figshare.14273234.v1. https://figshare.com/s/c7f5d0e75e096a20a8e1.

©2022 Raimi, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.