International Journal of
eISSN: 2576-4454


Research Article Volume 1 Issue 5
CSIR-National Geophysical Research Institute, India
Correspondence: Priyanka M, CSIR-National Geophysical Research Institute, India
Received: October 04, 2017 | Published: November 21, 2017
Citation: Priyanka M, Venkata RG, Ratnakar D. Groundwater quality appraisal and its hydrochemical characterization in and around Iron ore mine, Chitradurga, Karnataka. Int J Hydro. 2017;1(5):151-161. DOI: 10.15406/ijh.2017.01.00029
The characterization of different parameters, factors, and mechanisms controlling the geochemistry of groundwater with the help of several hydrochemical indices and comparison study of data were the aim of this paper. The groundwater quality status in and around the M/s. A. Narrain Iron Ore Mine, Chitradurga District, Karnataka has been investigated in the present study. Groundwater samples were collected for one hydrological cycle and analyses for major ion chemistry. Minerals dissolution, chemical speciation and water-rock interaction during the process of percolation under low precipitation and high evaporation condition and mining activities are major causes behind the major ion contamination and deterioration of groundwater quality in the studied region. Therefore, it became essential to examine the suitability of groundwater around the iron ore mining area for drinking and irrigation purpose. Hydrogeochemical indices including water Quality Index (WQI), modified Piper diagram, Gibb’s plot, Chloro-alkaline indices viz., CAI-I, CAI-II and SAR, RSC, KR ratio are used to assess the suitability of sample water for drinking, irrigation and domestic purpose. The analyses of CAI-I, CAI-II and SAR, RSC, KR hydrochemical indices confirm that groundwater of studied region is suitable for irrigation purpose. The analytical value of water quality index and comparison study of groundwater quality revealed approximately 60-70% samples exhibit poor water quality and slightly alkaline-fresh to brackish and chemically unsuitable for drinking purpose.
Keywords: CAI, Chloro Alkaline Indices; SAR, Sodium Absorption Ratio; RSC, Residual Sodium Carbonate; KR, Kelly’s Ratio; GW, Ground Water; WQI, Water Quality Index
Groundwater (GW) is a significant freshwater resource for domestic, industrial and irrigation water supply in the India. During last decades, the demand of groundwater supply for drinking, domestic, irrigation, industrial and multipurpose uses has increased due to the rapidly rising population and constant desire for a better lifestyle in developing countries. Increasing demand for fresh water supply is exerting tremendous pressure on both surface and groundwater reserves which leads toward overexploitation and unsustainable groundwater development. Unsustainable groundwater development, overexploitation, and improper management of mining waste, wastewater from mining areas causes adverse impact on quality of groundwater.1,2 However, the quality of GW is also directly or indirectly influenced by the leaching of geogenic contaminants through mining site as a consequence of rock-water interaction during the process of percolation 3 and when water moves through a hydrological cycle it transfers both inorganic and organic components from soil to water. Resulted, the higher concentration of ions in groundwater more than the acceptable limits of water quality standards4,5 which causes acute water-borne diseases in man and animals and also through food-chain.6,7 Therefore, the issues related to groundwater quality deterioration by anthropogenic activities are a pre-requisite for undertaking a detailed study on the groundwater geochemistry for restoring and maintaining its basic characteristics for which it is being explored. In Developing countries, mineral resources are significant sources for the economic development of the nation, but mineral development processes include exploration, extraction, and processing, are chief causes for poor groundwater quality in mining areas.8Exploration, extraction, and processing of minerals on a large-scale generate a huge amount of mining waste has become an environmental concern and responsible for several types of damage and hazards including groundwater pollution. Chemical speciation, oxidation, and dissolution of chemical agents of major water-bearing rock formations (granite, gneiss) and minerals found in the studied area like silicates, carbonates, dolomite, and hematite, limestone, calcite and pyrite, are major factors governing migration of geogenic pollutant.9,10 It has been reported that mobility, bioavailability, and leaching of major ions are also significant factors affecting the quality of groundwater. However, valence state, atomic weight, ionic potential and composition of soil are most important factors affecting the mobility of cations in the aqueous phase. Since, elements with higher atomic weight like Fe, Cd, Cr, and Zn have less mobility than major ions thus major ions are found in very high concentration than those of heavy metals.11 Soil with very less amount of clay and organic matter has very less sorption capacity and high mobility of ions from soil to groundwater.12−14 The hydrochemical processes like leaching of surficial salts, cation and anion exchanges, dissolution, precipitation and residential time all are major controlling factors for causing a change in the hydrochemical quality of groundwater of semi-arid and very dry areas having moisture indices less than minus 60%.15,16 The study on groundwater chemistry could reveal important information about geochemistry of groundwater, groundwater potential availability and the subsurface conditions of water-bearing rocks formations through which it is circulated.17‒21 However, characterization of hydrochemical properties of groundwater depends upon the several other factors involving mobility, chemical speciation, and hydrology, general geology and lithology of area, degree of susceptibility of rock formations to chemical weathering, recharge water quality, quantity and nature of point and non-point sources rather than the dissolution and water-rock interaction.22−25 The aim of the present study to characterization of different parameters, factors and mechanism which controls the groundwater quality in and around the iron ore mine and to predict its suitability and acceptability for drinking, domestic and irrigation purposes. The assessment and prediction of groundwater quality around iron ore mine have done through analysis of several physico-chemical paramters and by adopting various methods and calculation of statistical variants like Water Quality Index, Gibbs plots, Chloroalkali indices (CAI) Sodium Absorption Ratio (SAR), Residual Sodium Carbonate (RSC) and Kelly’s Ratio (KR). Comparision of analyzed data was made with the international and Indian standards.4,5
M/s. A. Narrain Iron Ore Mines of M/s. Vedanta Ltd. No. 2677 lies within14° 12’ 48.06” to 14° 14’ 08.00” N latitude and 76° 11’ 49.94” to 76° 12’ 54.93” E longitude with a total extent of 163.50 ha. The study area is located in between Megalahalli and Medakeripura villages of Chitradurga Taluk, Chitradurga District, Karnataka State with general ground elevation 891m above MSL. The key map of the watershed covering M/s. A. Narrain Iron Ore Mine, Chitradurga is shown in Figure 1. The climatic condition of the studied area can be described as seasonally dry, which experiences a hot summer (temperature 400C to 410C) and pleasant winter (temperature is around 140C to 170C). It receives low to moderate rainfall and is one of the drought-prone districts in the state. The average annual rainfall in the mining area varies 668mm to 457mm reported by.26
Geology and hydrology of the area
The area of mine is a part of Niruthadi Reserve Forest area located in between the villages of Megalahalli and Medakeripura, trending NNW to SSE. Topographically, the terrain in which the mine area is situated belongs to Dharwar system. The presence of crystalline schists, granitic-gneisses and the newer granites with few intrusive dykes has been observed in major geological rock formations of the study region. The structural investigation of study region has shown its soil cover is composed of red lateritic soil rich with various minerals as well as hematite (iron ore). Groundwater in the study area occurs within the weathered and fractured rocks under semi-confined conditions. The groundwater depth to the water level of all observation well varies from 11.38m to 39.47m (bgl) during pre-monsoon season and from 8.88m to 36.07m (bgl) during post-monsoon season.
Chemical analysis of water samples provides adequate information about qualitative and quantitative changes in groundwater systems which are useful to understand water quality conditions and effect of the composition of the material of aquifers and inputs of waters from various sources. The representative groundwater samples were collected in the vicinity of watershed covering of M/s. A. Narrain Iron Ore Mines for analyses of major ions during pre and post-monsoon period respectively. The location of collected samples is shown in Figure 1. The water samples were collected in polyethylene containers from 14 shallow and deep wells during pre and post-monsoon period. The sampling containers were washed with distilled water prior to collection and then washed again with respective sample water before sampling and closed airtight. Likewise, all samples were collected. The chemical (Na+, Ca2+, Mg2+, Cl-, F-, SO42-, HCO3-, NO3-) and physical (pH, TDS, EC) parameters for water samples were analyzed in the laboratory using the standard methods suggested by.27 The pH was measured by using the digital pH meter. Electrical Conductivity and Total Dissolve Solids were estimated by the HANNA EC and TDS analyzer. Electrical Conductivity and Total Dissolve Solids and pH of water provide information about the thermodynamic state of water.27 Sodium and potassium ions were analyzed by flame photometry using CL-345 flame photometer of ELICO. Sulphate was estimated by the turbidity method using UV-Vis spectrophotometer at 425nm wavelength. Fluoride was analyzed by colorimetric method using UV-Vis spectrophotometer at 570nm wavelength. Nitrate was analyzed applying the spectrophotometric method using UV-Vis spectrophotometer at 220nm wavelength. Standard titration methods were used to bicarbonate, calcium and chloride analysis. Furthermore, base-exchange indices (r1), meteoric genesis index (r2), Gibb’s Plot, modified Piper diagram, Chloroalkali indices- (CAI-I and CAI-II) graphs used to determine the all factors which are altering the geochemistry of groundwater and aquifer components and to understand its suitability for irrigation and domestic use.
Drinking purposes
Various physicochemical parameters such as pH, EC, TDS, Ca, Mg, Na, K, HCO3, SO4, and Cl of the collected samples were analyzed by various statistical methods. All statistical variations, minimum, maximum, mean, median and standard deviation of variables for both pre and post-monsoon period is presented in Table 1. The pH values of groundwater samples varied between 6.8 to 7.9 and 6.85 to 8.5 during the pre-monsoon and post-monsoon seasons (Figure 2). The result shows that none of the water samples of pre and post-monsoon seasons exceeded the pH value of the4−28 standards and also falls within the standard limits cited by Saudi Arabian Standards Organization,29 and U.S Environmental Protection Agency.30 The mean pH value of pre and post-monsoon sample water are 7.2 to 7.5 respectively (Table 1). If the water sample has pH value more than 7.0 is considered as alkaline. It indicates the presence of a few cations and bicarbonate ions in groundwater. The combined amount of all dissolve cations and anions in the water is expressed as Total Dissolved Solids (TDS). It does not include suspended sediments, colloids and dissolved gases. TDS varies from 78 mg/l to 1521 mg/l in pre-monsoon and 126mg/l to 900mg/l in the post-monsoon period in the watershed around Iron Ore Mine (Figure 2). The mean value of TDS is 666.71mg/l and 546.71mg/l for pre-monsoon and post-monsoon period respectively (Table 1). The high content of TDS in water responsible for the salinity of water and influences the utility of water for different purposes. It represents most of the samples of the studied region are found undesirable for drinking purposes (Table 2). Sodium is an essential element to regulate blood pressure for normal nerve and muscle functions and to maintain the fluid balance in human body. Sodium concentration ranged from 12 to 75mg/l during pre-monsoon period and 11 to 155mg/l during the post-monsoon period (Figure 2)(Table 1). The mean value of sodium concentration varied from 38.21mg/l to 65.19mg/l in pre and post-monsoon period (Table 1). Higher concentration of salts may induce physiological changes in the consumer. It ranges in between standard values.4‒28 Potassium is an essential element to regulate the metabolism in human being and to maintain the plant physiology. It varies from 08 to 10mg/l in pre-monsoon samples and 1.4 to 12.2mg/l in post-monsoon samples (Figure 2)(Table 1). Its elevated amount in drinking water effects on heart and kidney functions. Majority of samples have lower concentrations than standards value.4‒28 Calcium and magnesium both are the most common divalent cation of sample water. Both are principal cations responsible for the water hardness. The observed calcium ion concentrations were ranged between 21 to 220mg/l and 52 to 160mg/l during pre and post-monsoon period respectively (Figure 2). Most of the groundwater samples have higher Ca+2 concentration than permissible limits of >75mg/l as per4‒28 standards (Table1). Magnesium content varies from 24 to 112mg/l and 2.25 to 83mg/l in pre and post-monsoon period (Figure 2)(Table 1). The concentration of alkali metals in all water samples was higher during post-monsoon than pre-monsoon seasons. It may be because of areas lithology. The nitrate concentration in all samples of the studied region ranges from 98 to 517mg/l and 1.24 to 125 mg/l in pre and post-monsoon seasons respectively (Figure 2)(Table 1). Undesirable nitrate concentration > 45 mg/l as per4−28 standard was noted in approximately 100% pre-monsoon and 75% post-monsoon groundwater samples of the area (Figure 2). An abnormal concentration of nitrate in groundwater is resultant of the domestic wastewater discharge, and excessive fertilizer uses in or around the studied area. Higher concentration of nitrate in drinking water harmful to health causes blue baby syndrome. Bicarbonate concentration in both seasons’ samples was found less than the permissible limit 500mg/l specified by4‒28 (Figure 2 & Table 1). Hence, dilution of bicarbonate concentration by the rainfall recharge is obvious. Analytical result of Cl concentration shows that 80% water samples of post-monsoon seasons were found to be higher than those of pre-monsoon samples (Table 1)(Figure 2). Elevated Cl concentration has been observed in 25% post-monsoon samples are more than5 standard (Figure 2). Higher concentration of chloride produces a salty test in water. Cl contamination in arises in post-monsoon groundwater samples might be due to the local anthropogenic activities and runoff from agricultural areas after rainfall and discharge of human and animal waste in the studied area. Fluoride concentration ranged from 0.40 to 1.43mg/l during pre-monsoon season and 0.73 to 2.27mg/l during post-monsoon seasons (Figure 2)(Table 1). High Fluoride content was found in the groundwater of study area in post-monsoon seasons due to the breakdown and weathering of rocks such as granite and gneiss. Fluoride concentrations level between 0.8 and 1.0mg/l is essential for preventing the tooth from decay. However, its higher concentration than 1.0mg/l to 1.5mg/l,4−28 standard limit can cause skeletal fluorosis.
Parameter |
Maximum permissible |
Pre-monsoon |
|
Post-monsoon |
||||||||
|
|
Min |
Max |
Mean |
Med |
SD |
|
Min |
Max |
Mean |
Med |
SD |
pH |
6.5 to 8.5 |
6.8 |
7.9 |
7.28 |
7.3 |
0.35 |
6.85 |
8.25 |
7.75 |
7.885 |
0.42 |
|
TDS |
500 mg/l |
78 |
1521 |
666.71 |
585 |
360.03 |
126 |
900 |
546.71 |
537 |
192.13 |
|
Na |
200 mg/l |
12 |
75 |
38.21 |
36.5 |
14.51 |
11 |
155 |
65.19 |
65 |
35.47 |
|
K |
12 mg/l |
0.8 |
10.1 |
3.64 |
2.65 |
2.81 |
1.4 |
12.2 |
4.57 |
4 |
2.76 |
|
Ca |
75 mg/l |
21 |
220 |
101.21 |
103 |
49.27 |
52 |
160 |
100.86 |
100 |
29.23 |
|
Mg |
30 mg/l |
24 |
112 |
78.21 |
84.5 |
22.02 |
2.25 |
83 |
37.11 |
37.5 |
20.46 |
|
SO4 |
200 mg/l |
35 |
68 |
48.07 |
47.5 |
7.38 |
2.28 |
32.73 |
14.78 |
12.95 |
10.57 |
|
NO3 |
45 mg/l |
98 |
517 |
216.64 |
205.5 |
101.25 |
1.24 |
125 |
61.64 |
69 |
37.77 |
|
HCO3 |
500 mg/l |
73 |
468 |
321.07 |
353.5 |
120.31 |
31 |
294 |
176.71 |
170 |
63.27 |
|
Cl |
250 mg/l |
17 |
333 |
91.93 |
74 |
79.5 |
18 |
327 |
140.86 |
145.5 |
79.15 |
|
F |
1.5 mg/l |
0.4 |
1.43 |
1.24 |
1.28 |
0.25 |
|
0.73 |
2.27 |
1.27 |
1.08 |
0.47 |
Table 1 Statistical summary of physic-chemical parameters determined in pre and post-monsoon groundwater samples of M/s. A. Narrain Iron Ore Mine, Chitradurga
Min, Minimum; Max, Maximum; Med, Median; SD, Standard Deviation
Tds value (mg/l) |
Class |
Samples falling in dissimilar Seasons |
|
|
Pre-monsoon |
Post-monsoon |
|
No. of samples N=14 |
No. of samples N=14 |
||
< 500 |
fresh |
28% |
35% |
500-30,000 |
Brackish |
72% |
65% |
Table 2 Classification of ground water according to TDS value
Water quality index
Water quality index (WQI) of all water samples were calculated by the following expression.31,32
Vi, Estimated value of the ith water quality parameters of collected sample
Qi, Quality rating for the ithwater quality parameter
Si, Standard permissible value of the ithwater quality parameter (Table 3)
V0, Ideal value of the ithwater quality parameter in pure water (Table 3)
Wi, Unit weight for ithwater quality parameter;
K, Constant for proportionality
This index was developed by33 to determine the impact of the most common variables like pH, Electrical Conductivity (EC), Total Dissolved Solids (TDS), and Total Hardness (TH), Bicarbonate (HCO3), Calcium (Ca), Magnesium (Mg), Chloride (Cl), Sodium (Na), Fluoride (F), Sulphate (SO4) on water quality.34,35 Water quality rating based on WQI is described (Table 4). The analytical results of WQI of water samples are shown Table 5. The water quality rating of pre-monsoon sample water of study area is shown that 50% samples are unsuitable for drinking and other domestic use, while the rest 42% sample have very poor water quality (Table 5). 78% water samples collected during post-monsoon season fall in the category of very poor water quality rating. The complete analytical results (Table 5) are shown that groundwater samples of studied area are not suitable for drinking purpose.
Parameter |
Standards (Si) |
Recommended agency |
Ideal value (Vo) 32 |
pH |
6.5–8.5 |
BIS/WHO |
7 |
Total Dissolved Solids (TDS) |
500 |
BIS/WHO |
0 |
Sodium (Na) |
200 |
BIS |
0 |
Potassium (K) |
12 |
BIS |
0 |
Fluoride (F) |
1.5 |
BIS/WHO |
0 |
Chloride (Cl) |
250 |
BIS/WHO |
0 |
Calcium (Ca) |
75 |
BIS/WHO |
0 |
Magnesium (Mg) |
30 |
BIS |
0 |
Sulphate (SO4) |
150 |
BIS/WHO |
0 |
Nitrate (NO3) |
45 |
BIS/WHO |
0 |
Bicarbonate (HCO3) |
200 |
BIS/WHO |
0 |
Table 3 Drinking water standards recommending agencies and ideal value for all parameters (all concentration values except pH are in mg/l)
Wqi range |
Water quality rating |
0-25 |
Excellent water quality |
26-50 |
Good water quality |
51-75 |
Poor water quality |
76-100 |
Very Poor water quality |
>100 |
Unsuitable water quality |
Table 4 Water Quality Index (WQI) and rating of water quality
Sample No. |
WQI for Pre-monsoon samples |
WQI rating |
WQI for post-monsoon samples |
WQI rating |
N-1 |
99.33446 |
Very Poor |
84.10185 |
Very Poor |
N-2 |
113.1126 |
Unsuitable |
97.41986 |
Very Poor |
N-3 |
94.09373 |
Very Poor |
93.48763 |
Very Poor |
N-4 |
98.49496 |
Very Poor |
83.64454 |
Very Poor |
N-5 |
104.8226 |
Unsuitable |
79.21547 |
Very Poor |
N-6 |
66.79304 |
Poor |
75.61379 |
Poor |
N-7 |
105.5694 |
Unsuitable |
121.5685 |
Unsuitable |
N-8 |
103.1378 |
Unsuitable |
109.0722 |
Unsuitable |
N-9 |
99.23593 |
Very Poor |
127.2823 |
Unsuitable |
N-10 |
103.6824 |
Unsuitable |
93.96128 |
Very Poor |
N-11 |
98.08827 |
Very Poor |
89.53767 |
Very Poor |
N-12 |
97.97031 |
Very Poor |
89.1806 |
Very Poor |
N-13 |
102.1454 |
Unsuitable |
93.2903 |
Very Poor |
N-14 |
101.7176 |
Unsuitable |
92.0119 |
Very Poor |
Table 5 Water quality index and water quality rating for drinking purpose of all samples of both seasons
Irrigation water quality
The base-exchange indices r1 can be used to further classification of groundwater samples into two groups as Na+-HCO3- type and Na+-SO42- type by using the following Eq.1. If r1 value is >1 the groundwater samples will be categorized as Na+-HCO3- type while Na+ - SO42- type if r1<1.36
meq/l ……. (1)
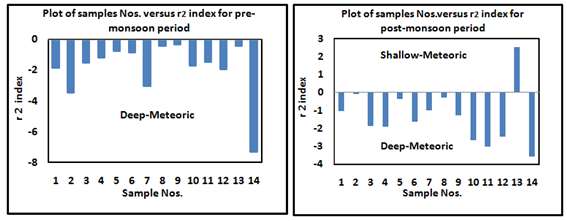
The result of base-exchange indices showed that all samples of pre-monsoon seasons belong to Na+-HCO3- type having r1 value >1 and most of the samples of the post-monsoon period except one belong to Na+-SO42- type, r1 value is <1 (Figure 3). Na+-HCO3- type water indicates the water is belonging to the industrial area, while Na+-SO42- type shows water sample is of marine origin and belongs from nearby coastline areas.37 Meteoric genesis index r2 can be used to determine the sources of groundwater samples are deep meteoric water percolation type or shallow meteoric water percolation type. Where r2< 1, the sources of groundwater are of deep meteoric water percolation type if, r2> 1 it shows the sources of groundwater is shallow meteoric water percolation type. Meteoric genesis index r2 can be computed by using Eq. 2.
meq/l …… (2)
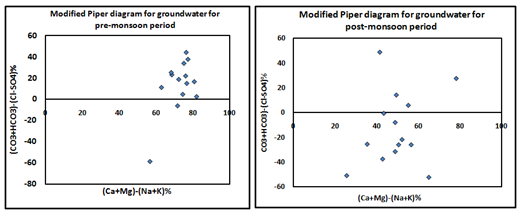
Results of these calculations confirmed that 100% samples of pre-monsoon season have deep meteoric water percolation type groundwater sources and except one, the all samples of post-monsoon period belong to deep meteoric water percolation type (Figure 4). Hydrogeochemical water classification using modified piper diagram (Chadha diagram) the classification for hydrogeochemical facies of water in terms of milliequivalent percentage differences between milliequivalent per liter of total dissolve alkaline earth and alkali metals (Ca+Mg)-(Na+K)%, and between milliequivalent per liter of total dissolve weak acidic anions and strong acidic anions (CO3+HCO3)-(Cl-SO4)%, was done by using modified Piper diagram (Chadha diagram). Modified Piper diagram (Chadha diagram) is modified version of Piper diagram38 and expended Durov diagram39 to predict the source of pollution in water. Modified piper diagram is useful to illustrate the geochemical evolution of groundwater by evaluating the chemical relationships among water type and milliequivalent percentage difference of dissolved major-ions on the basis of the position of data within the field of X-Y coordinate. According to Chadha diagram, the rectangular field of the plot can be divided into eight sub-fields. The results show that majority of the sample in pre-monsoon period belongs to the fifth group which indicates that Ca+Mg and CO3+HCO3 exceed Na+K and Cl-SO4 resultant temporary hardness was obtained in sample water (Figure 5A). Whereas, 65% sample of post-monsoon period belongs to group sixth, while 25% samples fall in 5th group (Figure 5B). It suggested that Na+K and Cl-SO4 ions concentration dominate over Ca+Mg and CO3+HCO3 in post-monsoon period samples.
Gibbs plots
Gibbs plot could reveal information on the mechanisms and processes which influence the geochemistry of water. Gibbs plot is used to determine the relationship between groundwater chemistry and lithological characteristics of the host rock or rock-water interaction. Gibbs ratio-I in which TDS versus is plotted for cations and TDS versus for anions in Gibbs ratio-II.40 This plot is categorized into three distinct fields on the basis of the position of data in the plotted area are evaporation dominance, rock dominance and precipitation dominance.41 The results of Gibbs ratio-I and Gibbs ratio-II are represented that all water samples of both seasons belong to evaporation dominance (Figure 6)(Figure 7). It demonstrates that evaporation is a major controlling factor which influencing groundwater quality through inducing the chemical weathering of host rocks of the study area.
Chloro-alkaline indices
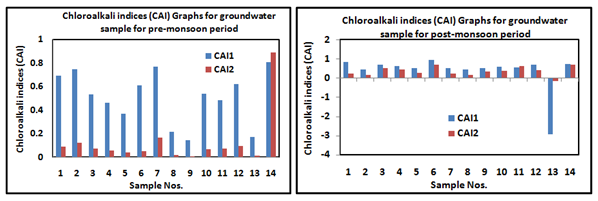
The CAIs is used to understand the changes in chemical composition of groundwater due to ion-exchange between groundwater, and it's sub-surface flow path through which it is circulated. The Chloro-alkaline indices can be evaluated by the formulae mentioned below (ions expressed in meq/l).42
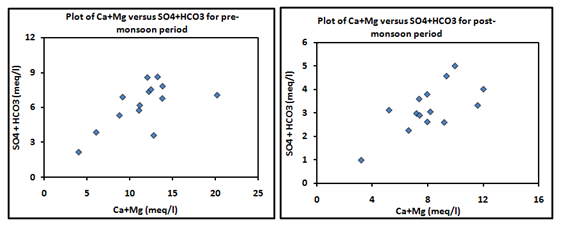
According to this formula, the ion-exchange process is classified into two equilibrium conditions are chloro-alkaline equilibrium and chloro-alkaline disequilibrium condition. Data analysis results of pre-monsoon and post-monsoon sample except one sample shows positive CAI indices ratio (Figure 8). The positive ratio is known as a base-exchange reaction, and resulting condition is called as chloro-alkaline equilibrium, whereas the negative ratio of ion-exchange is known as cation-anion exchange reaction and shows chloro-alkaline disequilibrium condition.43 Data points along the aquiline in the plots of Ca+Mg versus SO4+HCO3 (meq/l) for both seasons suggest carbonate weathering is a dominant phenomenon in the studied area. Figures show that most of the samples of pre-monsoon and 72% post-monsoon sample are affected by carbonate weathering while remaining by silicate weathering (Figure 9).
Kelly’s ratio
Kelly’s ratio is used to determine the suitability of water for irrigation purpose on the basis of the concentration of sodium ion against calcium and magnesium ions. Kelly’s ratio (KR) computed by the formula44,45 as given below: (all ionic concentration is expressed in meq/l).
Elevated sodium ion concentration is one of the prime concerns which causes salinity hazard in water and make it unsuitable for irrigation purpose. Based on Kelly’s ratio groundwater is classified into suitable, marginal and unsuitable if KR is <1, KR is 1-2, and KR is >2 respectively. All samples of both seasons are reported as suitable for irrigation has KR level less than 1 (Tables 6‒9).
Sodium absorption ratio
It is a significant parameter to determine the suitability of groundwater for irrigation purpose. It can be estimated by employing equation:
Water having the excess sodium absorption ratio >26 produces the undesirable effect on chemical composition of water, soil structure and reducing soil permeability by replacing absorbed calcium and magnesium46 The SAR values of pre-monsoon and post-monsoon seasons is founded <10. Hence, the water of study region can be graded as excellent for irrigation use (Table 7−9). In the equation sodium, calcium, and magnesium concentrations are expressed in meq/l.
Residual sodium carbonate
The presence of excess bicarbonate and carbonate ion concentration indicates water hardness and makes it unsuitable for agricultural purpose. RSC index <1 can be preferred as suitable for irrigation. It should not be >2 for irrigation water. Data analysis shows that values RSC index of studied water samples falls in the safe category and suitable for irrigation. The equation for calculating the Residual sodium carbonate (RSC) index is:
meq/l
According to RSC index groundwater for the agricultural purposes can be classified into three classes are suitable, where RSC is <1, marginal if RSC value between 1-2 and greater than 2 is graded as unsuitable (Table 8) (Table9).
Kr value |
Class |
Samples falling in dissimilar seasons |
|
(meq/l) |
|
Pre-monsoon |
Post-monsoon |
No. of samples N=14 |
No. of samples N=14 |
||
<1 |
Safe |
All Samples |
All Samples |
>1 |
Unsafe |
Nil |
Nil |
Table 6 Classification of groundwater quality for irrigation purpose based on KR values
SAR value (meq/l) |
Class |
Samples falling in dissimilar seasons |
||
Pre-monsoon |
Post-monsoon |
|||
No. of samples N=14 |
No. of samples N=14 |
|||
<10 |
Excellent |
All samples |
All samples |
|
18-Oct |
Good |
Nil |
Nil |
|
18-26 |
Fair |
Nil |
Nil |
|
>26 |
poor |
Nil |
Nil |
|
Table 7 Classification of groundwater based SAR values
RSC value (meq/l) |
Class |
Samples falling in dissimilar seasons |
|
Pre-monsoon |
Post-monsoon |
||
No. of samples N=14 |
No. of samples N=14 |
||
< 1.25 |
Safe |
All samples |
All samples |
1.25 –2.5 |
Marginal |
Nil |
Nil |
>2.5 |
Unsuitable |
Nil |
Nil |
Table 8 Classification of groundwater based on RSC values
Sample no. |
Pre-monsoon |
Post-monsoon |
||||
KR (meq/l) |
SAR (meq/l) |
RSC (meq/l) |
KR (meq/l) |
SAR (meq/l) |
RSC (meq/l) |
|
N-1 |
0.1 |
0.46 |
-6.26 |
0.11 |
0.34 |
-2.14 |
N-2 |
0.09 |
0.59 |
-14.23 |
0.32 |
1.39 |
-4.83 |
N-3 |
0.18 |
0.77 |
-3.27 |
0.31 |
1.32 |
-6.86 |
N-4 |
0.16 |
0.66 |
-4.39 |
0.31 |
1.17 |
-4.57 |
N-5 |
0.22 |
0.75 |
-3.07 |
0.33 |
1.2 |
-4.4 |
N-6 |
0.13 |
0.37 |
-2.82 |
0.15 |
0.38 |
-2.68 |
N-7 |
0.13 |
0.68 |
-8.09 |
0.38 |
1.7 |
-5.14 |
N-8 |
0.12 |
0.62 |
-5.59 |
0.28 |
1.08 |
-4.63 |
N-9 |
0.12 |
0.6 |
-4.38 |
0.46 |
1.87 |
-5.4 |
N-10 |
0.13 |
0.69 |
-7.07 |
0.32 |
1.58 |
-8.59 |
N-11 |
0.18 |
0.95 |
-5.6 |
0.58 |
2.8 |
-8.93 |
N-12 |
0.13 |
0.61 |
-5.96 |
0.27 |
1.07 |
-5.6 |
N-13 |
0.14 |
0.68 |
-5.83 |
0.4 |
1.61 |
-4.43 |
N-14 |
0.26 |
1.29 |
-10.59 |
0.38 |
1.47 |
-4.45 |
Table 9 Calculated parameters indexes of groundwater for irrigation quality
The groundwater quality analyses results indicate the groundwater around the M/s. A. Narrain Iron Ore Mines Chitradurga, Karnataka region is slightly alkaline and hard water. Results have revealed that the majority of samples have high Total Dissolved Solids value than permissible limit which make it unsuitable for the drinking purpose. The results of Base-exchange indices (r1) and (r2) and major divalent cations (Ca2+ and Mg2+) concentration has confirmed that sources of samples is deep meteoric percolation type which was common in semi-arid to arid region. Results of other standard classification methods are SAR, RSC and KR, Piper diagram, Gibbs plot and Chloro-alkali indices (CAI-I and CAI-II) showed that sample water is suitable for domestic as well as agricultural purpose. Furthermore, Result of all comparison study and water quality index are shown that water samples of the studied region are not fit for drinking. Groundwater samples have very high amount of sulphate, nitrate, and divalent cations. An elevated amount of nitrate ion in the sample water is indicated the presence of organic matter in the samples due to drainage of domestic effluent in the nearby area. Presence of very high concentrations of carbonates, oxides of divalent cations and sulphate indicates the exploitation of limestone, and pyrite, magnesite, silicates, and calcite minerals, and banded iron ore. Based on results it is found that exploration and processing of minerals caused moderate adverse impact on groundwater quality of iron ore mine area due to climatic conditions and improper management of mining area.47‒53
Authors are thankful to the Director, CSIR-NGRI, Hyderabad, for his kind permission to publish this manuscript. Authors are thankful to Sri Krishna Kulkarni AGM (HSE) M/s Vedanta Limited for initiation of the study and his detailed discussions throughout the study.
Authors declare there is no conflict of interest in publishing the article.

©2017 Priyanka, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.