International Journal of
eISSN: 2576-4454


Research Article Volume 6 Issue 2
1Universidade Federal do Triângulo Mineiro, Instituto de Ciências Biológicas e Naturais, Disciplina de Ecologia & Evolução, Brazil
2Centro Universitário de Belo Horizonte, Centro Universitário de Belo Horizonte, Brazil
3Universidade do Estado de Minas Gerais, Belo Horizonte/MG, Brazil
4Universidade Federal de Ouro Preto, Ouro Preto/MG, Brazil
Correspondence: Afonso Pelli, Universidade Federal do Triângulo Mineiro, Instituto de Ciências Biológicas e Naturais. Disciplina de Ecologia & Evolução, Brazil
Received: March 31, 2022 | Published: April 18, 2022
Citation: Camargo PRDS, Barreiros LFG, Barbosa NPU, et al. Golden mussel geographic distribution paradox: how can stream theories explain? Int J Hydro. 2022;6(2):73-77. DOI: 10.15406/ijh.2022.06.00304
Aquatic ecosystems have biological, social, and economic importance. Between the 1980s and 2000s, a few theoretical concepts emerged that attempt to provide a better understanding of the function and dynamics of freshwater ecosystems, including biotic and abiotic variables. The rationale for our research was based on observations of the distribution and abundance of an exotic bivalve mollusc, the golden mussel Limnoperna fortunei (Dunker, 1857). The species has been recorded from several river basins, but only occurs in large water bodies and fails to reach first-order streams. This study provides an overview of the main stream ecology concepts developed to explain the dynamics of lotic ecosystems in an attempt to solve this paradox. The river continuum concept was the first of many in river ecology. These theoretical concepts are not mutually exclusive, but interdependent. It is expected the main reasons for the non-occurrence of L. fortunei in small streams are twofold: fluctuations in physical and hydrologic conditions in small streams, generating instability, and reduced availability of plankton in first- and second-order streams.
Keywords: system dynamics, ecological interactions, aquatic ecosystems
Aquatic ecosystems including marine, estuarine, and freshwater environments support a rich species diversity. Aquatic biodiversity is believed to have originated in thermal waters during the Precambrian time.1 Aquatic organisms are distributed into five kingdoms: Monera, Protista, Plantae, Fungi, and Animalia.2 This diversity is responsible for the autochthonous support of aquatic ecosystems.3,4
In addition to their biological importance, freshwater ecosystems have social and economic importance and play an essential role in numerous activities including, among others,5,6 the establishment and development of cities, generation of hydropower, agriculture, livestock production, and fisheries.7‒10
Freshwater environments are classified based on their formation and physical, chemical, and environmental characteristics and often grouped into lentic (i.e., lakes and ponds) and lotic (i.e., streams and rivers) ecosystems.9 Lotic environments have an average annual flow of approximately 1 m³ s−¹ and are distributed over 7.56 million river kilometers and 508,000 km².10 Hydropower plants disrupt the natural flow of the river system, affecting its biotic and abiotic variables with the river no longer being considered a lotic environment, but a hybrid one with different regions of the reservoir showing differences in residence time.11,12
Stream ecologists have long recognized a need to better understand the dynamics of freshwater ecosystems, including the function, behavior, composition, and distribution of biological communities. Thus, between the 1980s and 2000s, a few theoretical concepts emerged that attempt to address these issues. Several theories have been developed, but only six gained wider recognition: the River Continuum Concept,13 Serial Discontinuity,14 Flood Pulse,15 Four-Dimensional Nature of Lotic Ecosystems,16 Process Domain Concept,17 and Uniqueness within the River Discontinuum.18
Their goal was to achieve an understanding of the structure and function of lotic ecosystems and enable stream ecologists to better predict the dynamics in river systems along the longitudinal, lateral, vertical, and temporal dimensions. However, accurately describing these systems is an extremely complex task and the studies often suffered from either a descriptive or predictive bias.19,20,21
Our research group has been conducting laboratory and field research on the golden mussel Limnoperna fortunei (Dunker, 1857), an invasive bivalve mollusc first introduced to South America in the early 1990s that is currently recorded from the South, Southeast, Central–West, and Northeast regions of Brazil.21 The rationale for this research was based on observations of the abundance and distribution of L. fortunei. For as-yet-unknown reasons and despite its wide geographic distribution, the golden mussel is mainly restricted to large water bodies, and often fails to reach and colonize first-order streams.
This study aimed to provide an overview of the main stream ecology concepts developed to explain and predict the dynamics of lotic ecosystems in an attempt to solve the golden mussel distribution paradox. Limnoperna fortunei is able to disperse to and colonize virtually every single river basin in Brazil. Current models predict that in the near future the golden mussel will be distributed across Brazil, but despite its huge biological potential, it does not colonize first-order streams. This review is an attempt to explain this pattern.
Using a structured search strategy, we searched the electronic databases Periódicos Capes, Google Scholar, Google Acadêmico, Scielo, and PubMed. Keywords used in the search were “River Continuum Concept”, “Serial Discontinuity Concept”, “Flood Pulse Concept”, “Four-Dimensional Concept”, “Process Domain Concept”, and “Uniqueness within the River Discontinuum” using the Boolean operator “AND”. Forty-three peer-reviewed papers published in English language only between 2016 and 2021 were included in the final review.
River continuum concept
The river continuum concept (Figure 1) recognizes that the physical variables within a lotic system present a continuous gradient of physical conditions from headwater to mouth. Along the length of a river, biotic and abiotic variables change in response to changes in habitat characteristics.13
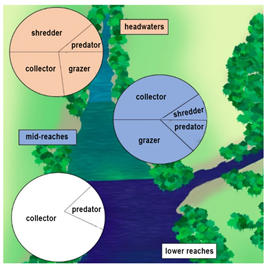
Figure 1 Stream communities are adapted to conform to changes in habitat characteristics as proposed by the River Continuum Concept.13
Biological communities are adapted to change (Figure 1) as the physical gradient changes.22,19 Headwaters are narrow and heavily influenced by riparian vegetation, which contributes large-scale inputs of allochthonous nutrients.23,24
In the mid-reaches of a river, allochthonous inputs become less important as river size increases and the importance of the terrestrial habitat is reduced.13 According to the authors, mid-reaches receive large quantities of nutrient transported from upstream. Moreover, mid-reaches are mostly dependant on autochthonous primary production. In the lower reaches further downstream, the river is wider and relies on nutrient inputs from upstream reaches and almost exclusively on autochthonous organic matter.25‒27
The river continuum concept was the first of many theoretical constructs developed in an attempt to explain the relationship between the biota and the environment of river–floodplain systems. These theoretical concepts of lotic ecosystems are not mutually exclusive, but interdependent. Nevertheless, the river continuum concept cannot be applied to veredas, which are unique wetland ecosystems often neglected in Brazil.44
Process domain concept
Aquatic ecosystems are heavily influenced by regional climate, geology, and topography, which control the geomorphic processes that are distributed over a landscape at a coarse scale.17 Thus, the process domain concept (Figure 2) recognizes that the structure and spatial composition of biological communities are largely governed by geomorphologic processes.28,29 A recent study30 showed that reservoir morphology, together with reservoir age and the position of the reservoir along the river, affect the composition and distribution of fish assemblages.
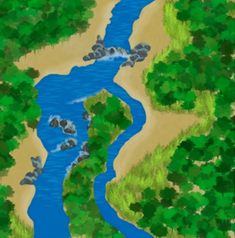
Figure 2 The geological and topological characteristics of the river affect the structure of biological communities as recognized by the Process Domain Concept.17
Flood pulse concept
A spectrum of hydrological and geomorphological conditions produces flood pulses (Figure 3) in river–floodplain systems.15 This theory recognizes that the flood pulse is the principal driving force for the productivity of biota and controls the exchange of nutrients between the river channel and the connected floodplain. Thus, the structure of aquatic communities and their ecological interactions are directly affected by seasonality.31
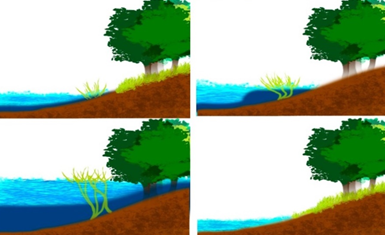
Figure 3 Seasonal fluctuations of the water level as recognized by the Flood Pulse Concept.15
The flood pulse may also potentially reduce interspecific competition for resources because of seasonal variations in hydrologic conditions.32 However, anthropogenic action such as installation of hydropower plants may alter the hydrological regime, affecting the aquatic biota.33
Serial discontinuity concept
The serial discontinuity concept14 recognizes that dams disrupt the river gradient, resulting in longitudinal changes in biotic and abiotic variables.34
Even though this concept received significant interest in the literature of lotic ecosystems (Figure 4), few studies have tested the applicability of the serial discontinuity concept.35‒38
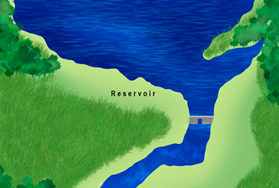
Figure 4 Dam construction disrupts the longitudinal and vertical continuum of the river system, resulting in changes in biotic and abiotic variables as predicted by the Serial Discontinuity Concept.14
Four-dimensional concept
The four-dimensional concept recognizes that the structure of lotic systems exists in a four-dimensional framework that includes lateral, vertical, longitudinal, and temporal dimensions. The lateral dimension includes interactions between the channel and floodplain systems as determined by seasonal hydrologic fluctuations. The vertical dimension incorporates interactions between the channel and the contiguous groundwaters.5 The longitudinal dimension integrates upstream–downstream interactions. Lastly, the temporal dimension includes interactions that occur on a geologic time scale.39
The four-dimensional concept (Figure 5) incorporates ideas from other stream ecology concepts, particularly the river continuum, flood pulse, and process domain concepts.40
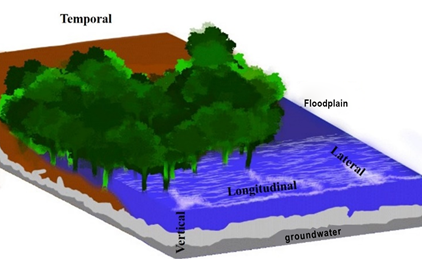
Figure 5 Schematic representation of the four river dimensions: longitudinal, lateral, vertical, and temporal.16
Uniqueness within the river discontinuum
Lotic ecosystems are comprised by habitat patches with different biotic and abiotic characteristics from the headwaters of small streams to the confluences of large floodplain rivers.41 River basin geomorphology can substantively influence longitudinal patterns of biological communities.42 The variation in biotic and abiotic variables across tributary streams (Figure 6) affects the structure of biological communities.41, 43 Thus, each river network can be described as a mosaic of habitat characteristics termed metastructure as proposed by the concept of uniqueness within the river discontinuum.18

Figure 6 Schematic representation of the confluence of two river basins and their distinct habitat characteristics termed metastructure as proposed by the concept of Uniqueness within the River Discontinuum.18
The theories considered here are susceptible to being accepted or refuted. It is worth noting that the choice for one or more of these theoretical concepts depends on the characteristics of the river system under study. Nevertheless, these concepts have, to a greater or lesser degree, limitations in their applicability.
The golden mussel fails to colonize headwater streams in the Triângulo Mineiro, southeast Brazil. One hypothesis is that headwater streams in the region are mostly vereda wetlands typically characterized by slightly acidic waters, which could be an obstacle to colonization by the golden mussel due to its cation exchange requirements (calcium ion Ca²+) for shell production and maintenance of vital processes.44,45 However, the same pattern has been reported from other river basins. High levels of humic and fulvic acids have also been detected in acidic headwater streams from other regions. These headwater streams naturally drain old, nutrient-poor soils. However, the golden mussel fails to colonize even headwater streams with suitable water conditions.
The golden mussel is widely distributed and has continuously expanded its nonnative range.46‒48 The species maintains viable populations at stream temperatures ranging from 10‒30 °C.
Limnoperna fortunei is planktonic, hence naturally nutrient-poor headwaters with low autochthonous production rates49,50 might not be able to support viable populations of the species, and this has been observed both in first- and second-order streams.
It is expected that the main reasons for the non-occurrence of L. fortunei in small streams are twofold. The first reason may be related to the plasticity of the species. Even though it can tolerate extreme environmental conditions, the golden mussel is unable to adapt to constant fluctuations in physical and hydrologic conditions as observed in small streams. Streams with an average flow below 0.5 L.s−¹ and that are not deeply incised show large temperature fluctuations. Thus, temperature instability could be a possible explanation for the non-occurrence of L. fortunei in small streams. Food availability is another major factor determining species distributions.
In conclusion, the ‘golden mussel distribution paradox’ can be explained by a lack of plasticity to adapt to fluctuating environments and the reduced availability of food and ions for cation exchange in small streams.
The authors thank the Institute of Biological and Natural Sciences at the Federal University of Triângulo Mineiro (ICBN/UFTM) and the Postgraduate Program in Environmental Science and Technology at the Federal University of Triângulo Mineiro (PPGCTA/UFTM). The authors also acknowledge the P&D UFOP/ANEEL GT-604 project for a scholarship managed by the Gorceix Foundation.
The author declares there is no conflict of interest.

©2022 Camargo, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.