International Journal of
eISSN: 2576-4454


Research Article Volume 5 Issue 5
1Graduate Program in Public Health and Environment and Department of Sanitation and Environmental Health, National School of Public Health, Oswaldo Cruz Foundation, Rio de Janeiro, Brazil
2Roberto Alcantara Gomes Biology Institute, State University of Rio de Janeiro, Rio de Janeiro, Brazil
3Laboratory of Environmental Health Assessment and Promotion, Oswaldo Cruz Institute, Oswaldo Cruz Foundation, Rio de Janeiro, Brazil
4Computational Biology and Systems Laboratory of the IOC / FIOCRUZ, Oswaldo Cruz Institute, Oswaldo Cruz Foundation, Rio de Janeiro, Brazil
Correspondence: Aloysio da Silva Ferrão Filho, Laboratory of Environmental Health Assessment and Promotion, Oswaldo Cruz Institute, Oswaldo Cruz Foundation, Rio de Janeiro, Brazil
Received: August 14, 2021 | Published: September 7, 2021
Citation: Sotero-Martins A, Carvajal E, Santos JAA, et al. Events linked to Geosmin and 2-methylisoborneol (2-MIB) in a Water Supply in the State of Rio de Janeiro, Brazil: a case study. Int J Hydro. 2021;5(5):214-220. DOI: 10.15406/ijh.2021.05.00283
Tastes and odors in tap water are problems faced by water companies worldwide, with consumers complaints mainly during summer, when cyanobacterial blooms occur and produce compounds such as geosmin and 2-methylisoborneol (2-MIB). We analyzed the data on taste and odor intensity and total concentration of geosmin and 2-MIB compounds in drinking water and raw water collected by the sanitation company supplying of the metropolitan region of Rio de Janeiro (Brazil) during the 2020 and 2021 water crises. Statistical and metagenomic analyses of the raw water samples of the year 2020, were performed. Organoleptic data allowed to signal the presence of these taste and odor (T&O) compounds in the drinking water, and the mean values of taste intensity were above the maximum allowed value of the Brazilian legislation, on average 37.5 times in 2020 and 5 times in 2021, indicating that the measures did not eliminate the problem. There was a linear correlation of 0.97 between the standard organoleptic taste and the total concentration of T&O in 2020. Metagenomic data, from raw water in the year 2020, for the mtf, mic and glys genes indicated 2-MIB as responsible for T&O. Modifications in the surveillance system of catchment and drinking water quality need to be adopted to circumvent the problems of cyanobacterial blooms in the Guandu basin, as conditions favorable to blooms will occur as long as the sanitation problems in this watershed are not solved.
Keywords: cyanobacteria, metagenomics, public health, geosmina, 2-methylisoborneol, tast and odour compounds
Eutrophication and freshwater pollution results in a dramatic increase in cyanobacterial biomass, which causes blooms.1 Blooms have a considerable economic impact on drinking water quality and safety, aquaculture productivity, and recreational activities in water bodies.2 Cyanobacterial blooms are a significant cause for water quality deterioration, causing deepwater hypoxia and anoxia, toxin production, food chain changes, fish kills, and unpleasant taste and odor events.3
Increased cyanobacterial proliferation and increasingly frequent episodes associated with geosmin and 2-MIB occurrence due to anthropogenic activities and climate change have led to global concerns about drinking water quality. Increasing awareness about the safe consumption of drinking water, aquaculture, or recreational water systems has increased the demand for a fast and robust on-site detection and monitoring system for geosmin and 2-MIB cyanobacterial events.4,5
The production of tast and odour (T&O) compounds, such as 2-methylisoborneol (MIB) and geosmin, by cyanobacteria, causing tastes and odors in tap water, are significant problems faced by water supply companies worldwide, with the number of consumer complaints highest during summer,4 thus undermining the safe and adequate supply of drinking water to the population.6 The sensitivity of humans to the odor of these compounds is very high (below 10 ng/L). In addition, these compounds are too stable to be metabolized.7 Although activated carbon treatment removes these compounds, this approach is costly and unsustainable for continued use.8 In addition, it is difficult to predict the amount of these compounds in the water from year to year because the odor intensity changes annually, even if the water condition remains similar. Therefore, predicting the occurrence of geosmin and 2-MIB in tap water to reduce consumer complaints, the cost of treatments to reduce the compounds, and decrease water insecurity is necessary.4
Geosmin and 2-MIB are volatile terpenes, sesquiterpene, and monoterpene, respectively. Geosmin biosynthesis has been described in actinomycetes and cyanobacteria, and the diphosphate sequiterpene is converted to geosmin by geosmin synthase.9,10 The genes encoding geosmin synthase have been identified in various organisms, such as Cyc2 in Streptomyces coelicolor; GeoA in S. svermitilis; NPUNMOD in Nostoc punctuforme.6,11,12 Many species of filamentous cyanobacteria have been confirmed as producers of geosmin and 2-MIB, such as: Anabaena, Planktithrix, Pseudanabaena (Planktonic), Phormidium, Oscillatoa, and Lyngbya.13,14,15,16
The Guandu Water Treatment Plant (WTP), located in Rio de Janeiro, belongs to the state company responsible for supplying 16 cities dependent on the Guandu System and 9 million inhabitants. This WTP collects raw water from the watershed completely eutrophicated by the untreated sewage load from upstream cities.17 Thus, in the summer, with the higher light intensity and lower water movement of the water in the catchment area, the growth of cyanobacteria is favored.18
The Brazilian legislation does not determine the maximum allowable values (MPV) for geosmin and 2-MIB in drinking water, but there are standard limits described for taste and odor.19 This legislation is currently under revision and proposals to include such parameters have been done. These data would provide a better evaluation of water quality and guide measures to be undertaken to prevent similar future water supply crisis.
The objective of this work was to analyze the data on organoleptic parameters and concentration of 2-MIB and geosmin in water captured from the Guandu Basin and the distribution network of water treated by the Guandu Water Treatment Plant (WTP), Rio de Janeiro, during the water crisis of 2020 and 2021. Raw water metagenomic data during the water crisis in 2020 allowed the evaluation of the cyanobacterial species that produced geosmin and 2-MIB in the Guandu Basin. With this data, the taste and odor data were correlated with the concentration of T&O compounds analyzed by the company.
Sampling location of the source's raw water
The water captured by the Guandu WTP (approximately 45 m3/s) comes from a catchment area formed by the rivers Ipiranga, Queimados, and Poços, resulting from the transfer of water from the Paraíba do Sul River (Figure 1), located between the municipalities of Nova Iguaçu and Seropédica in the State of Rio de Janeiro, Brazil (-22.80922:-43.62700). The catchment area covers an area of 350 km2.18 The water taken from this area enters the Guandu WTP and is treated by two stations: the old water treatment station (VETA), in operation since 1955, and the new station (NETA), inaugurated in 1982. This WTP is responsible for providing drinking water to a population of about 9 million inhabitants of the Rio de Janeiro state metropolitan region of 16 cities, dependent on the Guandu System.20 Human activities have changed the original regime of the watershed in the last 50 years. The most relevant factors are the increase in the population of the cities upstream, the increase of industries and agricultural activities along this watershed, without the necessary investments for basic sanitation.
Sample collection and processing in the year 2020
Samples of raw water from the Guandu Basin spring were collected during the crisis of the year 2020 in two moments. The first visit was on 01/13/2020, a time of great complaint from the population due to the taste and odor of the drinking water. In this first collection, a scarce volume of water (700 mL) was obtained from the WTP, under exceptional conditions with restricted access. This volume was ultracentrifuged (100,000 x g) for 2 hours at 4°C. DNA from the sediment was extracted using the DNEasy kit from Qiagen. The second visit to collect water from the spring was on 03/09/2020, a period with fewer complaints about taste and odor. In the second visit of 2020, 10 liters of raw water were collected and filtered in nitrocellulose membranes of 0.8 µm, 0.45 µm, and 0.22 µm of porosity. DNA extraction from these collections was performed with the "PowerWater® SterivexTM DNA Isolation Kit Sample" kit (MO bio laboratories, Inc). Library construction was performed with the Nextera DNA FLEX 2x150 bp paired-end kit from Illumina. DNA was quantified on Qubit™ DNA HS, and sample sizes were analyzed with a DNA Sensitivity bioanalyzer prior to sequencing. Sequencing was performed at the SENAI CETIQT Innovation Institute for Biosynthetic and Fibers sequencing facility (2020) using NextSeq 550 from Illumina (Illumina, INC, USA).
Obtaining secondary data for 2020 and 2021
The secondary data for the analyses of organoleptic parameters, taste, and odor, were obtained from public reports made available by the company responsible for water treatment, published on the electronic page https://www.cedae.com.br/relatoriosguandu, for the years 2020 and 2021,21 produced by a third-party company hired by the state company. Secondary data from three types of reports were analyzed. A time cut was made considering the periods of complaints about water quality, with reports of taste and odor in the water consumed by the population, namely: 1) taste and odor results from the distribution network analyzed by the production company; 2) taste and odor results from the treatment outputs (VETA and NETA); 3) geosmin and 2-MIB results, in total concentration (g/L) of the two compounds together at the Guandu WTP intake point and the average of the total concentration at the treatment output. However, for the year 2021, since the average value of the two water outlets of the WTP was not produced, the data described for VETA was used since we had no way to make the average value with the available data.
Analytical procedure of the metagenomic data
The sequenced data were analyzed on the Stingray@Galaxy platform.22 The quality of the sequenced samples was evaluated by FastQC 0.67.23 For data cleaning, Trimmommatic was used with cut-off above 38 and minimum length 20. The presence of the generated profiles related to the 2-MIB and geosmin sequences was evaluated. To assemble the profiles, protein sequences that had functional annotations for 2-MIB and geosmin were obtained from the RefSeq, UniProtKB, and Genbank databases of the National Center for Biotechnology Information (NCBI) version 3.5.0. Sequences were validated, and domains conserved in cyanobacteria were evaluated in NCBI CD-Search. The data were processed and analyzed in Stingray@Galaxy.22 FAST STASTIC version 1.0.0 was used for statistical verification. The median value of each file was considered for filtering the sequences by Filter sequences by length. The sequences of 2-MIB and geosmin were submitted to multiple alignments by the program MAFFT version 7.221.3.24 Profiles and similarity searches were generated in the program HMMER version 0.1.
Analytical procedure of secondary data
All mathematical and statistical calculations were done in Excel 2003 (Microsoft Office®) and BIOESTAT 5.3 (Mamirauá Institute). In determining the time period for considering the secondary data described in the CEDAE reports, the beginning of complaints of taste and odor in the water by the population of the municipalities supplied by the WTP was considered in the analysis up to 44 days after the complaint milestone date. After this period, the company initiated corrective measures to improve water quality, such as use of activated carbon and bentonite and ionically modified clay. Thus, for the year 2020, the starting date of the complaints was 01/04/2020, and for the year 2021, it was 01/19/2021. The maximum values found for the intensity of the parameters taste and odor at the collection points on the same day were used and quantified how many locations with sampling in the distribution network were performed by the company. Statistics were performed by Linear Correlation Test (Pearson) between the data of intensity of taste and odor and the total concentration of substances 2-MIB and geosmin, considering only the dates that had the organoleptic parameters equal to or greater than 6, which is the maximum permitted value (MPV) described in Brazilian legislation and when both parameters (taste and odor) were considered on the date analyzed.
Intensity data of the "Taste and Odor" parameters in the distribution network
In January 2020, cyanobacteria of the genus Planktothricoides were described as the most abundant and responsible for the production of substances that caused changes in the organoleptic pattern of the water produced at the Guandu WTP.17 For the same year, the results of the analysis of the intensity of taste and odor at points of the "Distribution Network" were presented only 40 days after the beginning of complaints by the population. That is, the company only started to analyze the taste and odor on 02/13/2020, so the data analysis cut-off was until 02/17/2020. In spite of that, just after 19 days of complaints by the population, the use of activated carbon and ionically modified clay was started in the water treatment stage at the Guandu WTP Figure 2 (A).
In January of the year 2021, during the summer period, with similar conditions of cyanobacterial blooms and due to the continued low sanitation conditions of the cities upstream of the Guandu WTP, the water supplied to the population of this hydrographic basin again presented a situation of non-compliance with the Brazilian legislation. During nine discontinuous but close days, the intensity of the parameter taste in the distribution network was above the maximum value allowed by the Brazilian legislation Figure 2(B). Although only on these days the "taste" parameter was equal to or above intensity 6, complaints from the population of different locations about the taste and odor in the water distributed were reported in the press during the whole period analysed, generating distrust about the quality of the water supplied. This fact led to an increase in the consumption of mineral water by the higher-income population. However, the low-income population had as their only alternative the reduction of water intake on days of "non-compliance" with Brazilian legislation,19 increasing public health risks in vulnerable groups.
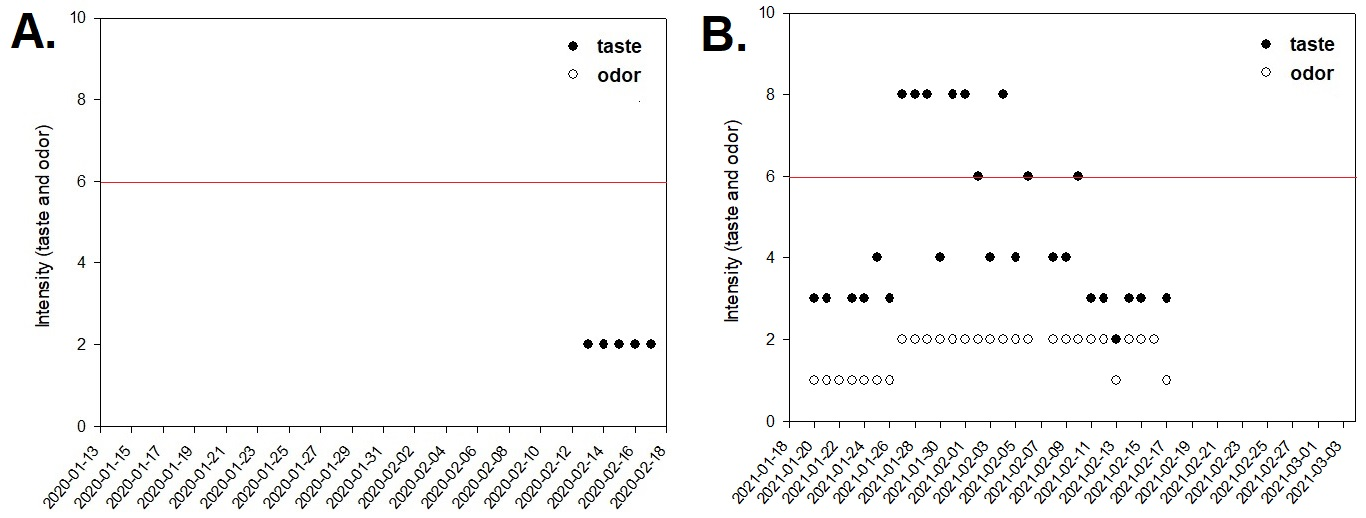
Figure 2 Data of the maximum intensity found for the parameters "Taste and Odor" in the Distribution Network for the five days in the 2020 crisis (A) and for the 29 days in the 2021 crisis (B), analyzed and presented in the tables available online. The red line represents the maximum permitted value (MPV) described in the Brazilian legislation.
On average, in the year 2020, collections were made at 13 points of assessment in the distribution network (SD = 10.3954), within the study cut-off period, characterized as a water crisis. However, there were days when 37 samplings were made and days when there was no sampling, or only two collections representing the monitoring day, and the median was nine collection points. In the year 2021, on average, there were 8.3 points of evaluation (SD = 5.9721), but also as in 2020, there was no pattern of monitoring, there were days with 24 samplings, and days without sampling or with a single point, and not always the same points were considered. Therefore, for the sake of standardization, we used the data of the maximum value found for each date.
In 2020, after 24 days of facing the situation of supply of drinking water with taste and odor, the intensity of the standard organoleptic taste, both in the output of the VETA and NETA, was well above the MPV Figure 3(A) described in the potability legislation.19 In the year 2021, only after seven days of complaint of the supply situation with taste and odor, the analyses of the contracted company were performed and showed values above the VMP Figure 3(B). However, when comparing the data from the NETA outlet with those from the VETA outlet, the water produced at VETA presented most of the time intensity of the parameters with higher values. In 2020, the maximum values for taste intensity at VETA and NETA was 67 and 17, respectively. In the year 2021, the amplitude of the data for VETA and NETA was 11 and 5, respectively. However, both treatment plants collect raw water from the same source.
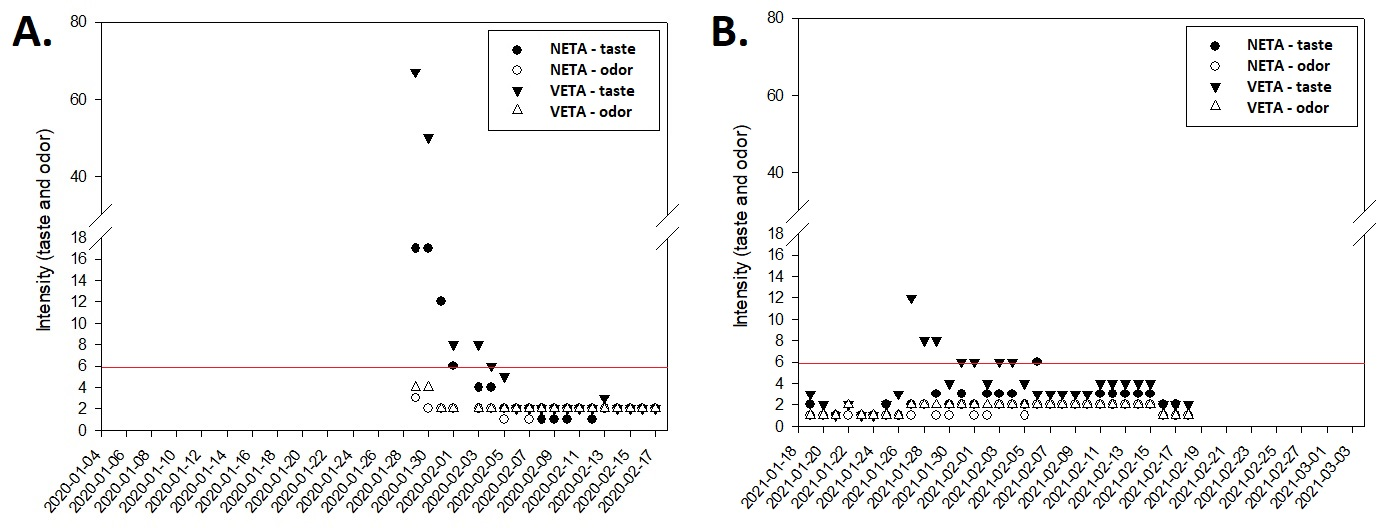
Figure 3 Monitoring of taste and odor parameters at the treatment output, comparison between NETA and VETA. The red line represents the maximum allowed value described in the Brazilian legislation, for the 20 days of monitoring in the 2020 crisis (A), and for the 30 days in the 2021 crisis (B), analyzed and presented in the tables available online. The red line represents the maximum permitted value (MPV) described in the Brazilian legislation.
Data on the substances geosmin and 2-MIB
The table with the data from the analyses by the company outsourced by CEDAE did not discriminate the individual values for each parameter, and only the total concentration is presented. Thus, when evaluating the total concentration of the substances 2-MIB / Geosmin at the point of catchment of raw water from the Guandu area, as well as at the treatment outlet, it is observed that the year 2020 was the year with the highest concentrations. However, these concentrations may have reached even higher concentrations since the analyses started to be done in 2020, only 23 days after complaints from the population, when the use of activated carbon inside the raw water intake tank at the Guandu WTP and ionically modified clay in the catchment area began to produce the expected effects, with the reduction of these compounds. Thus, in the following 20 days, the total concentration of these substances was decreasing both at the point of raw water intake and at the treatment outlet Figure 4(A). Although in the potability legislation,19 there are no reference values for these substances [Geosmin and 2-Methylisoborneol (MIB)], it can be suggested that they are responsible for the taste and odor parameters described in the Brazilian legislation. Therefore, the equivalence and correlation between these parameters are fundamental to support the monitoring since what is described in the legislation is qualitative. For the year 2021, even with the constant use of activated carbon, since 01/23/2020, in the raw water intake tank, as well as the use of ionically modified clay in the catchment area, the total concentrations of these compounds were only measured six days after complaints, so the population perceived changes in taste and odor, even with values below 10 ng/L Figure 4(B).
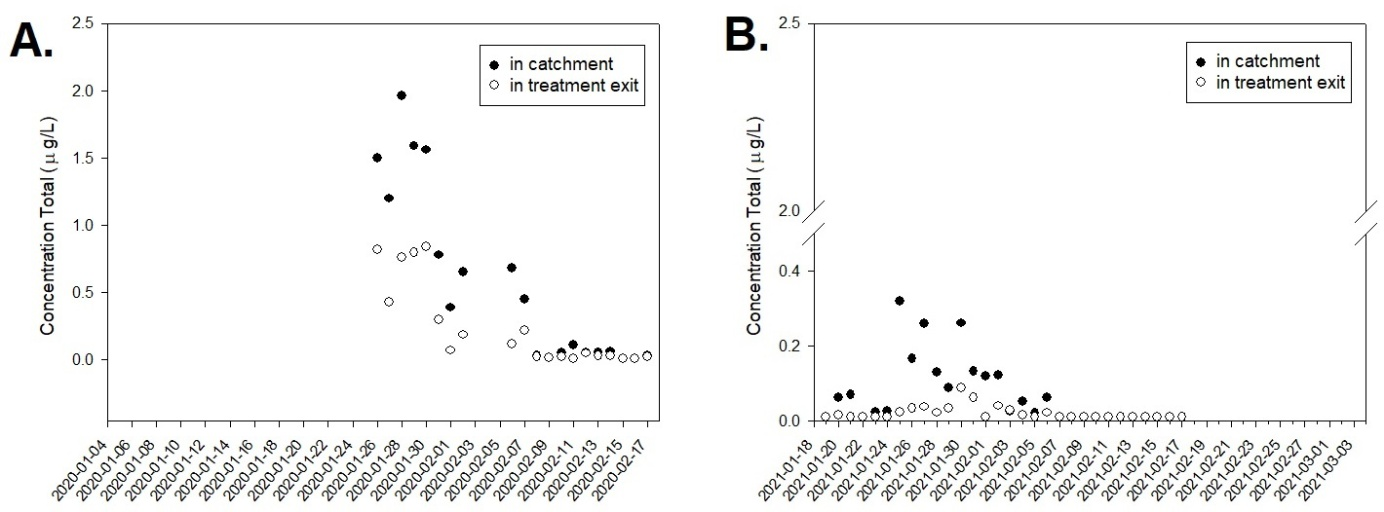
Figure 4 Monitoring of the total concentration of 2-MIB and Geosmin, in the Guandu System, within the period analyzed for the 23 days of the water crisis in the year 2020 (A) and 33 days in the year 2021 (B); at the raw water intake and treatment output points.
Correlation between the total concentration of 2-MIB and geosmin and the intensity of organoleptic parameters
It was only possible to apply Pearson's statistical correlation (r) between the intensity data of taste and odor and the total concentration of the substances 2-MIB and geosmin in the year 2020 because although there were only four days (01/29; 01/30; 01/31 and 02/01/2020) with intensity values above 6, there was variation in the values of the odor parameter. For the year 2021 there were 8 days (27/01; 28/01; 29/01; 31/01; 01/02; 02/02; 04/02; 06/02) with intensity values above 6, however the odor parameter did not vary, remaining with intensity 2, so Pearson's correlation was applied only between the intensity of taste and the total concentration of the substances 2-MIB and geosmin. For the data of the four days of a water crisis of the year 2020 with data above the MPV described in the Brazilian legislation, the average of these days was 37.5 for taste intensity (SD=26.16) and 3 for odor (SD=1.15), and the average of the total concentration of 2-MIB and geosmin was 0.503 g/L (SD=0.38). The linear correlation coefficient (r) between taste intensity and the total concentration of 2-MIB and geosmin substances was 0.95, and between odor intensity and the total concentration of 2-MIB and geosmin substances were 0.97. Therefore, these values indicate that there is a strong linear relationship between the variables.
However, for the year 2021, the mean values for the eight days of the water crisis in the year 2021, with data above the MPV described in the Brazilian legislation, were five times lower than those observed in 2020, with the mean for taste intensity being 7.5 (SD = 0.92), that of odor remaining constant at 2.0 and the mean of the total concentration of 2-MIB and geosmin was 0.023 g/L (SD=0.02). Linear correlation analysis between taste intensity and total concentration of 2-MIB and geosmin provided a value of r = - 0.012, i.e., there is practically no linear relationship between the variables.
Data on the number of reads found in the 2020 samples
In the comparative search with the sequences in the NCBI RefSeq and UniProtKB databases, 129 sequences were found for the 2-Methylisoborneol (2-MIB) biochemical pathway marker genes, which are the geranyl 2-methyltransferase diphosphate (GPPMT) and the MIB synthase (MIBS). And for the marker gene for geosmin synthesis, 1529 sequences were found for the marker gene Gys.
The index produced from the number of reads for the genes indicative of the metabolic pathways of 2-MIB and geosmin production, divided by the total number of reads in the raw water sample, collected in the middle of the January 2020 crisis was 6.42 times higher in the first collection (01/13/2020) than in the second collection (03/09/2020) for the geosmin synthase (GSG) gene related to geosmin. For the 2-MIB indicator genes, methyltransferase (mtf, also known as mib) and 2-MIB cyclase gene (mic), was 4.88 e-7, higher than that for geosmin indicator gene (Gys), which was 1.22 e-7 in the first collection, but it was not detected in the second collection.
In the treated water samples collected to be representative of distribution network, no sequences representative of the genes indicative of 2-MIB and geosmin were found.
In the past three decades, there is a gradual increase in reports of geosmin and 2-MIB in drinking water, and most of them have been associated with cyanobacterial proliferation.25 Due to the unpleasant taste and odor of earth and mud, it undercuts consumer confidence in the safety of treated water.26 This can be considered a public health problem because low water intake can compromise the human body's homeostasis, water balance, lowered immunity, and that the other deleterious effects of dehydration.27
Besides geosmin, 2-MIB is also a critical terpenoid responsible for altering organoleptic proprieties in drinking water, even at low concentrations.28 Chiu et al.29 reported that more than 40 species of cyanobacteria, including the genera of Pseudoanabaena, Planktothrix, Planktothricoides, Phormidium, Oscillatoria, and Lyngbya, had been reported as 2-MIB producers. Both geosmin and 2-MIB have very low odor threshold value (<10 ng / L) and is difficult to remove effectively using conventional water treatment processes.30,31 Furthermore, the lack of any clear pattern between geosmin / 2-MIB production and endogenous factors associated with cyanobacteria, as well as exogenous environmental factors (light, temperature, nutrient supply, and dissolved oxygen), makes it challenging to monitor and predict taste and odor events effectively.28
Among the various odorants identified, geosmin and 2-methylisoborneol (2-MIB), responsible for the musty or earthy odor, are considered the most commonly involved in drinking water quality alteration events.1,2,28 Other volatile organic compounds produced by cyanobacteria, such as sulfur compounds, carotenoid derivatives, fatty acid derivatives, amines, and terpenoids, are also associated with unpleasant taste and odor problems in water.2 Although they do not pose a risk to human health, these compounds cause insecurity and lack of confidence in the population, leading to complaints about water quality. In addition, they cause economic problems such as tourism and in the marketing of aquaculture products.32 Many cyanobacteria produce potent neurotoxins (anatoxins and saxitoxins) and hepatotoxins (microcystins, cylindrospermopsins, and nodularin) that pose serious health risks.1,33 Although these toxins can be present simultaneously as taste and odor compounds, no correlation between these compounds has been found in the literature that could justify the use of taste and odor compounds as indicators of the presence of toxins.34
In spite of that, other T&O episodes in the Guandu catchment area have occurred before indeed with the detection of toxins in raw water. In 2001, a similar crisis occurred, where the proliferation of cyanobacteria in Guandu made the taste of water treated by CEDAE unbearable. Contrary to recent episodes, a concentration of 0.4 g L-1 of microcystins was detected in the treated water and 0.32 g L-1 in the dialysis water at the Hemodialysis Center of the Federal University of Rio de Janeiro.35 Although this event did not lead to fatalities as the Caruaru Tragedy,36 blood samples analyzes of a group of 44 patients revealed hematological and biochemical alterations. This demonstrates how inefficient the Guandu WTP has been in removing these cyanobacterial compounds from the water and that there is always a potential risk of the presence of these toxins with cyanobacterial proliferation in water bodies used for drinking water supply.
These compounds, although they do not pose a risk to human health, cause insecurity and lack of confidence in the population, leading to complaints about water quality. Events also with limited sampling placed the Lake Mathews in California and in Missisippi, Izaguirre37 and Martin et al.,38 respectively on the CyanoGM Explorer scenario map (https://cyanogmexp.ncku.edu.tw) involving T&O production by cyanobacteria.
In our previous work, it was reported that the most abundant genus in raw water samples analyzed by metagenomics was Planktothricoides and the sequences for the most abundant species was SR001 strain of Planktothricoides sp.39 Metabolite profiling using a GC-MS/MS triple quadrupole system with automated SPME extraction showed that the strain SR001 of the genus Planktothricoides sp. produces 2-MIB but not geosmin.40 The same study reported that the strain SR001 do not produces microcystins and cylindrospermopsin, through enzyme-linked immunosorbent assay (ELISA).
In this study, the sequences, identified by metagenomics, were compared to the 129 sequences of the marker genes for the 2-MIB biochemical pathway and the 1529 sequences of the marker gene for geosmin synthesis. Knowledge about 2-MIB synthesis in cyanobacteria allows the development of molecular tools to monitor the production of taste and odor compounds related to the expression of the corresponding synthesis genes.29,41
Chiu et al.29 and Koltsidou42 suggest that geosmin production rate may be related to gene copy number and growth stage of the producing organisms. Su et al.43 obtained a quantification of the geosmin synthase gene (GSG) of Anabaena, in samples from different water sources in Beijing, China, using qPCR technique, and showed that significant correlation with geosmin concentrations of water samples measured using gas chromatography-mass spectrometry (GC-MS) analysis. Our study results corroborate with Su et al.43 as significant correlation was shown between taste and odor intensity with the average total concentration of 2-MIB and geosmin. Su et al.,43 as Moore,44 was focused on the possible expression of the geosmin synthase gene, and those involved with 2-MIB production. In Moore's44 work they developed a qPCR protocol focused on studying geosmin synthase gene expression under various environmental conditions, rather than assessing the T&O compounds production of these cyanobacteria.
Most current studies on geosmin and 2-MIB detection involve molecular biology techniques and the occurrence of target sequences for identification and detection of cyanobacteria involved with geosmin / 2-MIB production events offers advantages of detecting source organisms even at very low concentrations. Metagenomics allows the comparison and exploitation of direct sequences of organisms,45 and it is possible to explore and improve the characterization and detection of various groups of water contaminants of biological origin, and to analyze a set of metabolic genes of microbial communities, allowing to determine environmental conditions, such as pollution, and gene diversity.46,47
The data for the organoleptic, taste and odor parameters are available in public reports from the company responsible for water treatment, published on the website https://www.cedae.com.br/relatoriosguandu. And the raw water sequencing data that were evaluated in the metagenomics studies are in https://www.ncbi.nlm.nih.gov/bioproject/PRJNA738162/, from Comparative study of four CWS samples from Rio de Janeiro in 2020.
The analyses of the data made available by the treatment company indicate the need for a water safety plan and a standardization of sampling points in the distribution network that can be representative to signal what is happening in the water supply system. The lack of standardization in the periodicity of the sampling regime and the quantity of sampling points indicate a fragility in the monitoring system. Considering the strong positive correlation between the intensity of the organoleptic parameters, with the average concentration of 2-MIB and geosmin, detected by chemical methods, present both in the distribution network as well as in the treatment output, the intensity of the organoleptic parameters can be used as indicators of these taste and odor compounds. Furthermore, molecular biology methods, such as metagenomics analysis, can be used as a monitoring tool in pursuing genes related to the biochemical pathways of the biosysthesis of T&O compounds, making early detection of the presence of these compounds in drinking water possible.
We thank the Vice-Presidency of Environment, Care and Health Promotion (VPAAPS) of Fiocruz for funding, the Graduate in Program in Public Health and Environment at FIOCRUZ/ENSP, and the support of the Public Ministry of Rio de Janeiro, through the Specialized Environment Group (GAEMA) for the opportunity to develop this work. And the Coordination for the Improvement of Higher Education Personnel (CAPES). This study was partially supported by the Coordination for the Improvement of Higher Education Personnel (Coordination for the Improvement of Higher Education Personnel - CAPES) - Finance Code 001.
The authors have no conflicts of interest to declare.

©2021 Sotero-Martins, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.