International Journal of
eISSN: 2576-4454


Research Article Volume 3 Issue 5
1Division of Forensic Science, Galgotias University Greater Noida, India
2Department of Forensic Chemistry and Toxicology, College of Natural Sciences, Arba Minch University, Ethiopia
Correspondence: Mahipal Singh Sankhla, Division of Forensic Science, Galgotias University Greater Noida, India
Received: July 26, 2019 | Published: September 10, 2019
Citation: Sankhla MS, Kumar R, Biswas A. Dynamic nature of heavy metal toxicity in water and sediments of Ayad River with climatic change. Int J Hydro.2019;3(5):339-343. DOI: 10.15406/ijh.2019.03.00197
Heavy metal toxicity is major concern in term of water pollution. Heavy metals, some of them are potentially toxic and are transferred to the surrounding environment through different pathways. In this study, we carried out examination of concentration of Lead, Chromium, Nickel in water and sediment from River Ayad in Udaipur, Rajasthan, India by using Atomic Absorption Spectrophotometer. Samples of water and sediment were collected from the five different sampling sites. Samples collected in duration of ninety three days from January to April with the gap of 10-15 days during climatic changes are most. On comparison of these heavy metals concentration, it was found that concentration of Pb, Cr, and Ni were higher than the permissible limits of WHO and it increases with rising temperature and reducing humidity. The samples collected from Site 5 had higher concentration among all sites of collection.
Keywords: water pollution, toxicity, human health, water-borne disease.
Water is a major source of life. Contamination of water is the major threat in today’s world. Water is an important part of human and animal life and its depends upon life cycle and presence of whole bio-diversity. People cannot make or produce either of these basic fundamentals of life in a form in which persons are wanted. As such people have no right to abolish, waste with any natural resources. Wherever and in whatever those form may be establish, it is our basic duty to preserve such natural resources. Water quality has developed a severe issue due to growing industrial development Toxic waste, urbanization. The constituent existing in the water organizations depend on the nature where the water body is located and the release value from several sources in that water body.1 Udaipur is the city of lakes; there are many water bodies, lakes and rivers. The Ayad River is the main river of Udaipur city which passes through the heart of the city. It is one of the ancient rivers in the history of Mewar which has been long quenching the publics of Udaipur. The spill water of the well-known Pichola and Fateh Sagar Lake of Udaipur district gets into the Ayad River. But due to constant anthropogenic interfering or rather misuse, today it is much contaminated. It conveys all domestic waste water from urban housing and reimbursement and industrial waste water from industrial pockets of Udaipur. All the domestic waste water and industrialized waste water finally terminates into Udai Sagar Lake. Though ironically, this historically significant river is at current working as the drainage body of the Udaipur city in Ayad River occupied with sewage and garbage. The river movements through the Udaipur city and is its higher drainage body.2 Heavy metals can also be loosely defined as a subset of elements that exhibit metal properties. It contains the transition metals, some metalloids, lanthanides, and actinides. Using density as a defining factor, also distinct heavy metals as those taking a exact density of more than 5g/cm3.3 Heavy metals covering farming runoff enter in aquatic atmosphere, it may toxic to aquatic plants and animals. If compostable waste such as sewage sludge, municipal solid waste and pig manure contain heavy metals, it may modification the composting procedure by preventing bacterial growth. In the vermicomposting procedure heavy metals affects earth worm life cycle.4 Heavy metals establish one of the most hazardous groups because of their insistent nature, toxicity, affinity to collect in organisms and experience food chain magnification and more still, they are non-degradable. Water bodies may develop polluted by the growth of heavy metals and metalloids through discharges from the quickly expanding industrial areas, disposal of high metal wastes, leaded gasoline and paints, land application of fertilizers, animal manures, sewage sludge, pesticides, wastewater irrigation, and Electronic waste. There are several studies which infer that there is change in water environment in terms of concentration of heavy metals during climatic change.5,6 This had been noted that dissolved heavy metals concentration are more in rise in environmental temperature as compare to cold weather. Heavy metal poisonousness has established to be a major risk and there is several health threats related with human and animal. The toxic effects of these metals, even though they do not have any organic role, persist current in approximately or the other form damaging for the people body and its suitable working. Consequently, the aim of the present study was to measure the concentration of heavy metals from the Ayad River during winters and summers to appreciate the change in dissolved heavy metal concentrations.
Samples collection
The water samples were collected from the five different Sites of Ayad River at Udaipur, Rajasthan, India. Site 1:- Pula, Site 2:-Ayad Puliya, Site 3:-Sevasharm, Site 4:- Sector 3; Site 5:-Sukha Naka (Figure 1).
All sampling sites were used for Farming and drinking and considered to be more polluted due to human activities.Water samples were collected for analysis from each Site. All samples were collected in 1.5liter of sterile polyethylene bottles, which were pre-washed with 10% nitric acid and de-ionized water. Before sampling, the bottles were rinsed at least three times with water from the sampling site. The bottles were immersed to about 20cm below the water surface to prevent contamination of heavy metals from air. Sediment Samples were also collected for analysis from each site. All samples were collected in sterile polyethylene bag.
All water samples were immediately brought to the laboratory where they filtered through Whatman No.41 (0.45μm pore size) filter paper. The samples were acidified with 2ml concentrated Nitric acid to prevent precipitation of metals, reduce adsorption of the analyses onto the walls of containers and to avoid microbial activity, then water samples were stored at 4°C until the analyses.
Digestion of sediment samples
For extraction of heavy metal from sediments, the standard method described by American Public Health Association, toxicological manual was followed.7 Three representative subsamples each of about 1g of dried soil was digested with 15ml of a 5:1:1mixture of nitric acid, sulphuric acid and perchloric acid in water bath maintained at 800C until a transparent solution was obtained. After cooling, the solutions were filtered through Whatman filter paper and diluted to 100ml with de-ionized water.8
Instrumentation
The concentrations of heavy metals were determined in all samples by Atomic Absorption Spectroscopy (Element AS AAS4141). It is a standard laboratory analytical tool for metal analysis and is based on the absorption of electromagnetic radiation by atoms.
The concentration of lead, nickel and chromium concentration in water and sediment collected in every 10-15days during four months from January to April 2017 from Ayad river were measured and compared with the permissible limits as set by the World Health Organization (WHO).
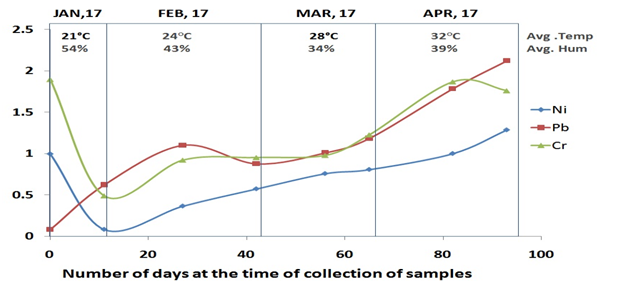
Figure 2 Concentration of Lead, Nickel and Chromium in water in different month with reference to temperature and humidity (Avg, Average; Hum, Humidity; Ni, Nickel; Pb, Leab; Cr, Chromium).
Concentration of heavy metals in water samples
Lead (Pb): In month of January, the concentration of lead (Pb) in water was 0.351ppm followed by sudden rise in February (0.989ppm) which further increased in March (1.092ppm) and continue to increase in month of April as where the concentration of lead in water was 1.995 ppm. Maximum permissible limit of Lead as per the WHO guideline is 0.01ppm. With comparison to this limit, the concentration of lead is very high than the permissible limit, it may cause in severe toxicity of Lead in human being as well as in animals. The level of lead in April month is almost 200times higher than limit given by WHO (Figure 1). There is increase in dissolved heavy metals with the increase in climate temperature and decrease in humidity. There is significant difference between the WHO limit and levels measured during these months. On comparison of the levels among these months, we find significant differences in concentration of Pb in different months (Table 1).
Nickel (Ni): In month of January the concentration of nickel (Ni) in water samples was 0.538 followed by slight decrease in February concentration was 0.468, whereas there was increase in the March (0.782) and further it increases in month of April, the concentration of nickel in water was 1.143. According to the WHO guidelines, maximum permissible limit of nickel is 0.02ppm. We found that concentration of nickel is very high as compared to the permissible limit, and almost 60times higher than WHO limit in month of April. There were significant differences between the concentration of WHO limit and Ni levels measured during these months. On comparison of the concentration of Ni among the different months, we found significant differences in concentration of Ni in different months (Table 1).
Chromium (Cr): In month of January the concentration of chromium (Cr) in water is 1.192ppm followed by February concentration of chromium is 0.935ppm and in the month of March, the concentration is 1.10ppm and in month of April is 1.81ppm. The WHO guideline for maximum permissible limit of chromium level is 0.001ppm. The concentration of chromium in Ayad river water sample is higher than the permissible limit, and it is almost 1800times higher than the permissible limit in the month of April. There is significant difference between the WHO limit and Ni levels measured during these months. On comparison of the levels among these months, we find significant differences in concentration of Pb in different months (Figures 2&3).
Concentration of heavy metals in sediment samples
Lead (Pb): The concentration measured from the sediments in the month of January was 19.013ppm followed by steep increases by February, the concentration of Lead was 33.308ppm. In the month of March, the concentration of dissolved Pb increases to 42.046ppm and small increase was further noted in month of April (48.988ppm). The concentration of Lead in Ayad river sediment sample is higher than the permissible limit (10ppm), and it is almost 4times higher than the permissible limit in the month of March and April. There is significant difference between the WHO limit and Ni levels measured during these months. On comparison of the levels among these months, we also find significant differences in concentration of Pb in different months (Table 1).
Nickel (Ni): The concentration of nickel in sediments was found to be 32.001ppm in samples collected in January. There is increase in concentration by February and measured 38.360ppm where steep increase was noticed in the month of March and April, the concentration was 44.815ppm and 60.034ppm respectively. The concentration of nickel in Ayad river sediment sample is higher than the permissible limit (20ppm), and it is almost 3times higher than the permissible limit in the month of April. There is significant difference between the WHO limit and Ni levels measured during these months. On comparison of the levels among these months, we find significant differences in concentration of Ni in different months (Table 1).
Chromium (Cr): In month of January the concentration of Chromium in sediment was 38.764ppm, which increases in month of February (47.595ppm) and in the month of March, the concentration further increases abruptly to 69.428ppm and continued to month of April to 81.515ppm. The concentration of Chromium in Ayad river sediment sample is higher than the permissible limit (25ppm), and it is almost 3times higher than the permissible limit in the month of April. There is significant difference between the WHO limit and Ni levels measured during these months. On comparison of the levels among these months, we find significant differences in concentration of Pb in different months (Table 1).
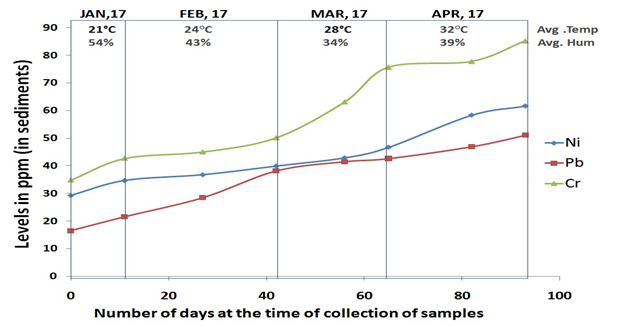
Figure 3 Concentration of Lead, Nickel and Chromium in sediment in different months with reference to temperature and humidity (Avg, Average; Hum, Humidity; Ni, Nickel; Pb, Leab; Cr, Chromium).
Climatic changes and level of heavy metals in water and sediments
As we can infer from the, that January being coldest month in the four months, with average temperature of 21oC and there is increase in temperature with months ahead of year. On comparison, we found there is a consistent increase in level of heavy metals with temperature rise. There is on average of 4oC change in every month from January to April, and there is change in concentration of heavy metal in both sediments and water. In water samples, we find a sudden drop in concentration levels of heavy metals in month of January and then there is constant increase in concentration of heavy metals with rise in temperature. For the sediments, we found that there is continuous increase of concentrations level of heavy metals. We had also observed similar kind of pattern with the humidity level of environment, as the humidity level decreases with rise of temperatures, there is increase in level of concentration of heavy metals. This dynamic nature of concentration levels of heavy metals in Ayad River with respect to climate change should be accountable and considered for further stratifying the preventative measures.
In term of the different sites of collection, we found that Site 5 (Sukha Naka) had more concentration than any other sites of collection for most of the samples for both water and sediments, whereas others sites are most nearby the average (Table 1). Although Site 3 had low concentration of Cr compare to five sites of sample collection (Table 1).
|
Collection sites |
Conc of Pb (ppm) |
Conc of Ni (ppm) |
Conc. of Cr (ppm) |
|||
|
Water |
Sediment |
Water |
Sediment |
Water |
Sediment |
|
|
Site 1 |
0.798 |
36.929 |
0.627 |
42.625 |
1.066 |
61.199 |
|
Site 2 |
0.825 |
39.098 |
0.560 |
41.870 |
1.231 |
59.398 |
|
Site 3 |
1.155 |
33.373 |
0.771 |
46.321 |
1.022 |
54.448 |
|
Site 4 |
1.313 |
31.665 |
0.853 |
50.064 |
1.372 |
55.843 |
|
Site 5 |
1.402 |
39.15 |
0.855 |
38.752 |
1.612 |
67.334 |
Table 1 Concentration of Heavy metals (Pb, Ni, Cr) in water and sediments collected from different sites of Riven Ayad, Rajasthan, India
Max, Bold; Min, Italic;
Heavy metals are found in various water body and it is harmful for both aquatic life as well as human life. There are many studies related to heavy metal toxicity in previous literature which time to time reported the levels of heavy metals in water bodies. WHO had strictly suggested the permissible limit but most of water bodies are contaminated with polluted water released from industry and nearby factories. On account of the research of the drinking water samples, contain Heavy metal concentration more than the admissible and desirable levels (WHO). Most of the water samples were at highly contaminated, which are not possible to use for drinking purposes. The toxicologists have continually detected the heavy metal concentration in various water bodies. The rise in the elemental pollution makes water and fish not suitable for consumption, which may cause severe health problems.
Ayad River is the main water bodies in Udaipur city from which water is supplied to most of the households. The heavy metal toxicity in river bodies is serious problem and should be addressed by environmental protection leaders. In this study, we found the most of water and sediment samples collected from 5sites had higher concentration as per the WHO limits, which means there is continuous exposure of this river with heavy metal contamination through various sources. In Ayad River, previous study reported Pb concentration (1ppm), and showed improved total dissolved solids (TDS) and biological oxygen demand (BOD) may recommend increased organic matter (OM) within the river from industrial release, contaminated water and wastes and ability of self-purification.2 In a study, it was found that the recorded concentration of Pb was 0.06ppm9 while in present study, the Pb concentration was very high (1.099ppm), for Chromium concentration in water is 0.14ppm10 while in our research we found Chromium concentration in water as 1.261 and for Nickel concentration in water is 1.02ppm10 while in our research we found Nickel concertation in water 0.733ppm. Lead concertation in sediment is 25.1ppm11 while in our research we found Pb concentration in sediment 35.839ppm, for Cr concentration in sediment is 58.40ppm12 while in our research we found Chromium concertation in sediment 59.325ppm and for Nickel concertation in sediment is 67.08ppm12 while in our research we found Nickel concertation in sediment 43.803 ppm. One of the largest rivers in India, Ganga, also reported to be higher levels of heavy metals concentrations.13 These heavy metals are toxic and enters in environment may lead to bioaccumulation and biomagnifications. This increase in heavy metals concentration is related to industrial waste discharges which are released to water bodies’ untreated leading to increase in these toxic heavy metals concentrations. In present study, this is noted by the data evaluted on the basis of different sites, site 5 had higher concentration among the 5sites, as we located most of industrial discharges are nearby site 5.
In one of the study conducted in Malaysia in water tributaries found that there is increase in dissolved heavy metals pre-monsoon as compared to post monsoon.5 another study also suggested increase of concentration of heavy metals in summers and less humid enviornement.14 the possible reason for the increase in concentration is reduced water levels during summer or increase in temperature. There are also many government plans are going to clean the Ayad river.15
The concentrations of heavy metals have already crossed or are at the borderline of the permissible limit as declared by World health Organization in most of river bodies. Although the some previous data suggests that somewhere the elemental concentrations is still below the permissible limit. Human health is directly affected by the consumption of polluted water, sediment, fishes, fruits, vegetables, plants etc. Studies show that Industrial wastes, E- waste, Sewage, Natural source, anthropogenic source, and Agricultural actions that have contaminated dangerous and toxic constituents in the Ayad River water thereby, led to pollution of drinking water in near areas. Diseases like Neurotoxicity, Carcinogenicity basically related to contamination of heavy metals in water such as Pb, Cr and Ni are also prevalent in such areas. The practice of trace element detection should be continued to lower possible consumption of contaminated eatables. People should be aware of the hazardous effects of consumption of polluted water and eatables.
None.
The authors declare that there are no conflicts of interest.
None.

©2019 Sankhla, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.