International Journal of
eISSN: 2576-4454


Research Article Volume 8 Issue 6
Doctor in Veterinary Sciences, Master in Biodiversity and Restoration of Natural Spaces, Argentina
Correspondence: Pablo Guillermo Rimoldi, Chair of Biology and Ecology, Faculty of Veterinary Sciences, National University of Rosario (FCV-UNR). Ovidio Lagos Boulevard and National Route 33, Casilda, Santa Fe, CP. 2170, Argentina
Received: November 20, 2024 | Published: December 6, 2024
Citation: Rimoldi PG. Diversity and composition of a micromammal assemblage in Southern Santa Fé province, Argentina. Int J Hydro. 2024;8(6):223-228. DOI: 10.15406/ijh.2024.08.00393
Species diversity is a central issue in both community ecology and conservation biology and diet analysis of Tyto furcata has proven to be a tool of high methodological value in determining the presence and distribution of micromammals. The objective of this work is to present the diversity, composition and relative abundance of the species that conform the assembly of micromammals in four environments in the South of Santa Fé province, Argentina. It was obtained a total of 8132 prey recovered from 3442 pellets. From the results obtained, it was possible to establish a specific richness (S) for the study area of 20 species of micromammals, among them four species of sigmodontine rodents not documented for the study area: Oligoryzomys nigripes, Calomys venustus, Holochilus chacarius and Graomys chacoensis. This work shows that although the ansembly of micromammals is typical of the Pampean ecoregion, the most complex (vertical variation) and heterogeneous (horizontal variation) environments that are still preserved present species diversity, since they contain greater diversity of microhabitats.
Keywords: Argentina, Ecology, micromammal, Santa Fé
Species diversity is a crucial aspect in community ecology and conservation biology, especially because of their vulnerability to human activities.1 In Argentina, agricultural expansion and population growth have profoundly transformed the ecosystems of the Pampas region, the most extensive grassland system in the country, with an area of approximately 540,000 km², distributed among Buenos Aires, La Pampa, Córdoba, Santa Fé and Entre Ríos.2
These biomes have undergone severe modifications due to agriculture and cattle ranching, leaving natural remnants in areas unsuitable for production.3 Agricultural transformations affect ecological processes such as population dynamics and community structure, with variable impacts on mammals depending on their spatial and dietary needs and their ability to adapt to anthropized landscapes.4
While some species have declined or disappeared, others, such as rodents, have thrived thanks to the generation of new habitats and abundant food resources. This has facilitated the global expansion of species such as Mus musculus and rats (Rattus rattus and Rattus norvegicus).5 Furthermore, in recent decades, studies on rodents in Argentina have increased, highlighting their economic and sanitary relevance as agricultural pests or disease reservoirs.6
In the Pampas region, particularly in Buenos Aires, rodent communities are among the best documented in the country.7 However, knowledge about micromammals in Southern Santa Fé is limited and fragmented, with imprecise spatial and temporal data. This work seeks to expand the mastofaunistic information of this area, analyzing the diversity of micromammals (≤ 1000 g) through the study of the prey of the Barn Owl (Tyto furcata).
Study area
The study was conducted in the Casilda district, South of the province of Santa Fé, head of the Caseros department. It is located between 33° 02′ 39″ South latitude and 61° 10′ 05″ West longitude (Figure 1).
The total area is 38.400 hectares (384 km²) of which 1,200 hectares correspond to urban area and the remaining 37,200 hectares to rural area, thus becoming the dominant matrix of the landscape.
Given that this institutional framework defines the activity, and that anthropic activity is the factor that modifies the support, the type of land use permitted is the factor that imposes new conditions on the territory and, consequently, on the ecosystems. For this reason and for the purposes of this work, the criterion of type of land use was adopted for the characterization of the territory determined as the study area and thus four (04) types of environments belonging to land ecosystems were established.
Data collection
The analysis of pellets, pellets regurgitated by birds of prey, allows us to study the distribution, abundance and vulnerability of prey species, as well as to discover new species or extend known distributions. This work used diet analysis of Tyto furcata to evaluate the micromammal fauna in environments of the Casilda district. Between January and December 2023, pellets were collected monthly at established points. All available material was taken after cleaning the perches, ensuring that each sample corresponded to the recent period. The pellets were stored in labeled bags, complying with biosecurity measures, and then dried in the laboratory at 70°C for 48 hours. They were then measured, weighed and processed to extract mandibles and skulls using surgical instruments. The compacted pellets were soaked beforehand. The skeletal remains were identified by comparison with osteological collections and specialized literature. Each pair of mandibles or skull of the same species was counted as an individual.
Methods for measuring diversity
Total diversity (gamma diversity) was estimated following Halffter & Moreno,8 who define it as the number of species in the set of sites or communities that make up the landscape, in this case, the number of species recorded in the different environments present in the study area.
The observed and estimated species accumulation curves (rarefaction) were calculated for each environment using the StimateS 8.2 program. The estimators calculated were: Chao-2, ICE, Jacknife 1, Jacknife 2 and Bootstrap. Although the expected values generated by the estimators can be used as measures of alpha diversity, in this work they were used to determine how effective the sampling was.9
For the association of micromammals, it was determined: (a) species richness (S), understood as the number of species in a sample; (b) relative abundance, understood as the percentage fraction of the total number of animals,10 which allowed us to identify species of low representativeness (low abundance); (c) diversity α (intra-environment), considering specific richness and structure. The latter was determined according to Shannon and Wiener's diversity index, which quantifies the total diversity of a sample, being influenced by two fundamental components: richness and equity. It thus considers the importance value of each species and expresses the uniformity of importance values across all species in the sample. The formula for this function is: H'= -Σ (pi x log2 pi), where pi is the proportion of the total number of individuals in the sample that corresponds to the species, whose values are displayed between zero when there is only one species, and the maximum (H'max) corresponding to log2 S. In addition, the Pielou equity index (J) was calculated according to the equation: J= H'/H'max. This index quantifies the contribution of equity to the total observed diversity.
Their values fluctuate between 0 and 1, so that 1 corresponds to situations where all species are equally abundant.11 To test the null hypothesis that the diversity H' of the three environments are equal, the procedure of Hutcheson12 described in Zar (1996) was followed, consisting of a t-test calculating the weighted diversity index (Hp = (NlogN)-(Σfi log fi)/N).
This paper also presents the use of effective number (of species) as a measure of diversity to compare ecological communities (alpha diversity) García-Morales et al.,13 To estimate diversity, the three Hill numbers14 were calculated as a measure of diversity components.15 Hill numbers are obtained with the formula:
where qD is the true diversity,16 pi is the relative abundance of species i (proportional abundance), S refers to the number of species comprising the community and q is the order of diversity.16 The exponent q modulates the sensitivity of the index to the relative abundances of species. In a way q determines how many species are considered within the analyzed sample, according to their rarity,14 giving, as it increases from 0 to 2, less and less weight to rare species and more weight to the most abundant ones. For example, as mentioned in Beninato,15 zero-order diversity (q = 0) is insensitive to species abundances; thus it is simply equivalent to species richness (0D) (Moreno et al., 2011). When q = 1, it behaves like Shannon's exponential index (1D), weighting the frequency of species, without disfavoring rare or uncommon species. Finally, when q = 2, it behaves as the inverse of Simpson's index (2D), weighting species with higher relative abundance.14,15
The degree of similarity in species composition between the different environmental units was estimated using the Jaccard index.11 The dissimilarity in species composition between pairs of biotas (Colwell and Coddington)17 was obtained from the complementarity analysis. This ranges from zero, when both sites are identical in species composition, to one, when species from both sites are completely different.17 To establish the beta diversity for the total study area, the Whittaker index modified to a percentage was used. This index, besides being the most widely used in beta diversity studies, has proven to be the most robust for measuring replacement between communities.11
A total of 8132 prey items recovered from 3442 pellets were obtained. Of the total, 97.7% belonged to micromammals, 91% being native rodents of the family Cricetidae, followed by introduced rodents of the family Muridae n=633 (7.96%). The lowest proportion of prey was represented by the families Caviidae n= 61 (0.76), Molossidae n=8 (0.1%) and juveniles of Leporidae n=2 (0.025%).
From the obtained results, it was possible to establish a specific richness (S) for the study area of 20 species of micromammals. The species recorded were: Lutreolina crassicaudata (Desmarest, 1804), Monodelphis dimidiata (Wagner, 1847), Eumops bonariensis (Peters, 1874), Molossus molossus (Pallas, 1766), Tadarida brasiliensis (I. Geoffroy Saint-Hilaire, 1824), Akodon azarae (Fischer, 1829), Oligoryzomys flavescens (Waterhouse, 1837) Oligoryzomys nigripes (Olfers, 1818) Calomys musculinus (Thomas, 1913), Calomys laucha (Fischer, 1814), Calomys venustus (Thomas, 1894), Holochilus chacarius (Thomas, 1906), Necromys lasiurus (Lund, 1840), Oxymycterus rufus (Fischer, 1814), Graomys chacoensis (J. A. Allen, 1901), Cavia aperea (Erxleben, 1777), Rattus norvegicus (Berkenhout, 1769), Rattus rattus (Linnaeus, 1758) Mus domesticus (Schwarz and Schwarz, 1953) and Lepus europaeus (Pallas, 1778). For each species, the type of environment where the record was made and its relative abundance modified to percentage are indicated in order to visualize the dominant species in each type of environment (Table 1, Figure 2).
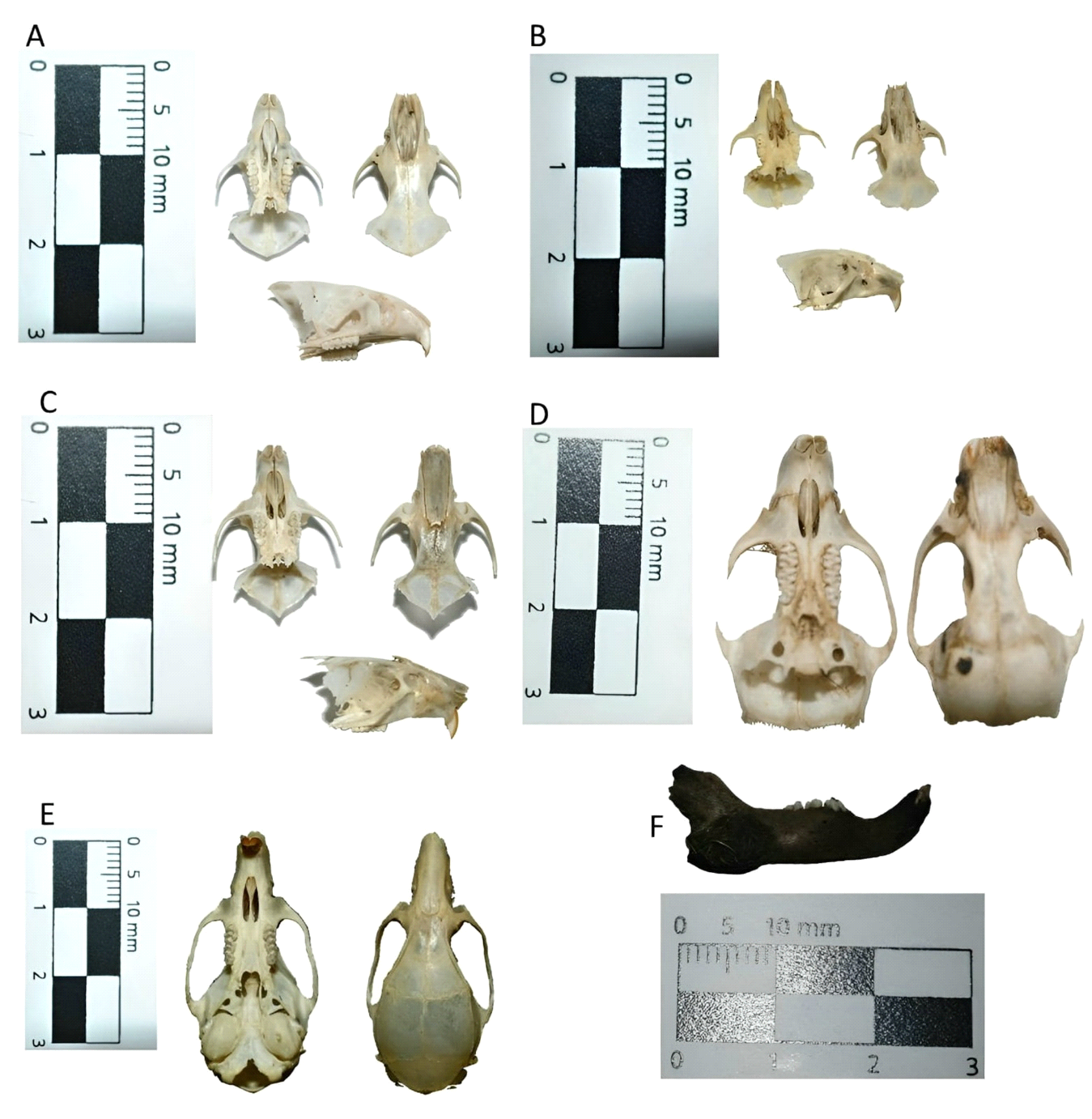
Figure 2 Some cranial elements that make up art of the results. References: A- Akodon azarae; B- Calomys sp.; C- Oligoryzomys sp.; D- Holochilus chacarius; E- Mus domesticus; F- Lepus europaeus.
|
Species |
ARNAN |
AP/S |
ARA |
AU |
||||
|
ni |
pi (%) |
ni |
pi (%) |
ni |
pi (%) |
ni |
pi (%) |
|
|
DIDELPHIMORPHIA |
||||||||
|
Didelphidae |
||||||||
|
Lutreolina crassicaudata |
2 |
0,11 |
- |
- |
- |
- |
- |
- |
|
Monodelphis dimidiata |
1 |
0,06 |
- |
- |
- |
- |
- |
- |
|
CHIROPTERA |
||||||||
|
Molossidae |
||||||||
|
Eumops bonariensis |
1 |
0,06 |
2 |
0,12 |
- |
- |
- |
- |
|
Tadarida brasiliensis |
2 |
0,11 |
2 |
0,12 |
- |
- |
- |
- |
|
Molossus molossus |
1 |
0,06 |
- |
- |
- |
- |
- |
- |
|
RODENTIA |
||||||||
|
Cricetidae |
||||||||
|
Akodon azarae |
612 |
35,17 |
480 |
28,02 |
1218 |
29,96 |
- |
- |
|
Oligoryzomys flavescens |
483 |
27,76 |
280 |
16,35 |
641 |
15,77 |
20 |
4,69 |
|
Oligoryzomys nigripes |
27 |
1,55 |
- |
- |
- |
- |
- |
- |
|
Calomys cf. C. laucha - C. musculinus |
497 |
28,56 |
862 |
50,32 |
1994 |
49,05 |
9 |
2,11 |
|
Calomys venustus |
17 |
0,98 |
- |
- |
- |
- |
- |
- |
|
Holochilus chacarius |
22 |
1,26 |
- |
- |
- |
- |
- |
- |
|
Necromys lasiurus |
24 |
1,38 |
16 |
0,93 |
- |
- |
- |
- |
|
Oxymycterus rufus |
15 |
0,86 |
- |
- |
- |
- |
- |
- |
|
Graomys cf. Chacoensis |
20 |
1,15 |
- |
- |
- |
- |
- |
- |
|
Caviidae |
||||||||
|
Cavia aperea |
14 |
0,80 |
13 |
0,76 |
34 |
0,84 |
- |
- |
|
Muridae |
||||||||
|
Rattus norvergicus |
- |
- |
6 |
0,35 |
13 |
0,32 |
12 |
2,82 |
|
Rattus rattus |
- |
- |
28 |
1,63 |
21 |
0,52 |
38 |
8,92 |
|
Mus musculus |
- |
- |
24 |
1,40 |
144 |
3,54 |
347 |
81,46 |
|
LAGOMORPHA |
||||||||
|
Leporidae |
||||||||
|
Lepus europaeus |
2 |
0,11 |
- |
- |
- |
- |
- |
- |
|
Total number of individuals (N) |
1740 |
1713 |
4065 |
426 |
||||
|
Total number of species (S) |
16 |
10 |
7 |
5 |
||||
Table 1 List of micromammals by type of environment surveyed in the Casilda District, southern Santa Fe province and their relative abundance. References: ARNAN: Non-anthropized rural or natural environment; AP/S: Peri-urban/suburban environment; ARA: Anthropized rural environment; AU: Urban environment
Based on the behavior of the diversity estimators for each of the environments, it seems unlikely to obtain a greater number of species than those collected even if the sampling effort was increased, since the species accumulation curves stabilized or tended to decrease.
According to the Shannon-Wiener index (H') (Table 2), the non-anthropized or natural rural environment (ARNAN) showed the highest diversity (H'= 1.47) with an H'max= 2.7 followed by the peri-urban/suburban environment (AP/S) with an H'= 1.24 and with an H'max= 2.30. In third place is the anthropized rural environment (ARA) with (H'= 1.20) with an H'max= 1.94. Last in terms of biodiversity is the urban type environment with (H'=0.71) with an H'max= 1.60.
|
Environments |
Shannon Whiener Index |
True Diversity |
||
|
oD |
1D |
2D |
||
|
ARNAN |
1,47 |
16 |
4,36 |
3,53 |
|
AP/S |
1,24 |
10 |
3,45 |
2,78 |
|
ARA |
1,2 |
7 |
3,34 |
2,8 |
|
AU |
0,71 |
5 |
2,03 |
1,48 |
Table 2 Results of the diversity analysis. Shannon-Whiener Index values and observed effective number values are indicated. Abbreviations as in Table 1
Regarding Pielou's equity index (J), the values show intermediate values of uniformity with ranges of J= 0.44 for the urban environment, J=0.53 for the non-anthropized or natural rural environment and the periurban/suburban environment, and finally J=0.61 for the anthropized rural environment.
In terms of diversity (H') there are significant differences in three of the four environments studied. Between the non-anthropized or natural rural environment and the periurban/suburban environment (t0.05(2) 3440 =1.96), between the non-anthropized or natural rural environment and the anthropized rural environment (t0.05(2) 2721 =1.96), between rural non-anthropized or natural type environment and urban environment (t0.05(2) 608.55 = 1.96), between peri-urban/suburban environment and urban environment (t0.05(2) 583.79 = 1.96), between rural anthropized environment and urban environment (t0.05(2) 473.34 =1.96). The t values obtained are higher than the t values in table (7.17 for non-anthropized or natural rural type environment and periurban/suburban environment, 10.21 for non-anthropized or natural rural and anthropized rural environment, 13.28 for non-anthropized or natural rural type and urban environment, 9.38 for periurban/suburban environment, 9.38 for non-anthropized or natural rural type and urban environment, 9.38 for periurban/suburban environment and urban environment, and 9.15 for anthropized rural environment and urban environment), so the null hypothesis is rejected and it is concluded that the diversity of micromammals is not equal among the three environments described.
Contrary to this situation occurs when comparing the periurban/suburban environment and the anthropized rural environment (t0.05(2) 2825.05 =1.96) with a t obtained of 1.59 showing that there are no significant differences between the two assemblages in terms of diversity (H').
With respect to the values of effective numbers observed (Table 2) it can be mentioned that the richness (q = 0) clearly differentiates the non-anthropized or natural rural environment (ARNAN) as the richest environment in micromammal species (16 species). When all species and their relative abundance were included in the diversity measure (q = 1), the same trend as that obtained with the Shannon-Wiener index was found. Diversity decreased from the non-anthropized rural or natural environment (ARNAN) to the urban environment (AU). By expressing these trends between the two most dissimilar environments, it can be established that the rural non-anthropized or natural environment (ARNAN) is 2.15 times more diverse in micromammal species than the urban environment (AU). In other words, the urban environment (AU) has only 49% of the diversity of the natural environment (ARMAN).
If the two most biodiverse environments (ARNAN and AP/S) are compared, the results show that the non-anthropized or natural rural environment (ARNAN) is 1.26 times more diverse than the periurban/suburban (AP/S), with the latter having 79% of the representation of the ARNAN assemblage. This trend is similarly reflected when comparing the non-anthropized or natural rural environment (ARNAN) with the anthropized rural environment (ARA). In general terms, the two environments with a medium degree of anthropization (AP/S and ARA) are very similar in terms of diversity, but always with the (AP/S) environment above the (ARA). This propensity changes when analyzing the measure of diversity of order 2 (q = 2). In this case, although the non-anthropized rural or natural environment continues to be the most biodiverse, the anthropized rural type environment (ARA) takes second place, displacing the peri-urban/suburban type environment (AP/S). The order 2 diversity measure gives greater weight to common species, providing information about dominant species. Between two communities, it indicates a greater equity in the distribution of abundances among common species. This result agrees with the Pielou (J) equity which positioned the rural anthropized environment as the most equitable in terms of relative abundance compared to the peri-urban/suburban (PA/S) environment.
When analyzing the similarity and complementarity between environments in terms of the micromammals surveyed, important variations can be observed in terms of species composition. Beta diversity among environments present in the study area, measured by Jaccard's coefficient of similarity (Bold) and complementarity (italics). Abbreviations as in Table 1.
|
Environments |
Rural non-anthropized or natural |
Periurban/Suburban |
Anthropized rural |
Urban |
|
RNAN |
- |
0,36 |
0,21 |
0,1 |
|
P/S |
0,63 |
- |
0,7 |
0,5 |
|
RA |
0,78 |
0,3 |
- |
0,7 |
|
U |
0,89 |
0,5 |
0,28 |
- |
With respect to Whittaker's beta diversity for the entire study area, the result obtained was 1.90, this expressed as a percentage11 represents 90.47%, which indicates a high beta factor.
The biological communities are characterized by properties such as diversity, composition, and the relative abundance of the species that comprise them.18 These characteristics vary depending on the scale at which they are analyzed, as community structure results from processes operating at different spatial levels.19 At a regional scale, landscapes dominated by agriculture tend to be homogeneous. However, at a local level, the inclusion of urbanized areas, agricultural and livestock fields, natural patches, and transitional zones increases the heterogeneity of the environment. Species perceive these dynamics differently depending on their ecological requirements and dispersal capacities.20 Although the general trend is a reduction in diversity and abundance for some species, others manage to increase their populations due to new niche opportunities or the decline of predators and natural regulators.21 This study employed the analysis of Tyto furcata pellets as an indirect method to assess the diversity and composition of small mammal communities in the Casilda District. This approach is based on the assumption that owl predation accurately reflects the distribution and abundance of their prey.22
The results provided novel insights into the local small mammal fauna in a region scarcely explored from a mastozoological perspective. This work reports for the first time the presence of Monodelphis dimidiata in the area, as well as range extensions for four sigmodontine rodents (Oligoryzomys nigripes, Calomys venustus, Holochilus chacarius, and Graomys chacoensis), confirming previous observations.6,22,23 In anthropized rural environments (ARA) and peri-urban/suburban areas (AP/S), the community was dominated by Calomys cf. C. laucha - C. musculinus, a typical pattern in regional agroecosystems.24 However, in non-anthropized rural environments (ARNAN), Akodon azarae became dominant, with an increased presence of Oligoryzomys flavescens, indicating their preference for less disturbed habitats with greater vegetation cover.22 These environments, being more complex and diverse, offer a greater variety of microhabitats, facilitating the coexistence of specialized species like Holochilus chacarius and Oxymycterus rufus. On the other hand, in urban areas (AU), the dominant species was Mus domesticus, representing more than 80% of the community. This, along with the low equitability index (Pielou J=0.44), indicates a strong biological homogenization, which is concerning due to the role of this species as a reservoir for diseases such as leptospirosis.25
Anthropization level was the main structuring factor for the studied communities. Less disturbed environments (ARNAN) hosted a greater diversity of native species, while the most altered areas (AU) were dominated by exotic species, consistent with global patterns of faunal replacement. The diversity analysis (Shannon-Wiener) confirmed this trend, with ARNAN being the most diverse environment, although the effective species index showed some variations favoring ARA. Complementarity analysis between biotas revealed significant differences between ARNAN and the more anthropized environments. The high beta-diversity (90.47%) suggests the presence of species with restricted distributions, highlighting the importance of conserving less altered environments. This study expands local records and lays the foundation for future monitoring focused on managing species of sanitary and economic interest. Moreover, it emphasizes the value of Tyto furcata diet analysis as an effective tool for assessing small mammals, avoiding invasive methods such as intensive trapping, which are more costly and pose greater sanitary risks.
None.
The author declares there is no conflict of interest.

©2024 Rimoldi. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.
 World Water Day is celebrated globally on March 22nd. Water is the most essential source for sustaining
life, and it plays an important role in the well-being of every human being. From the International Journal of Hydrology, we wish to convey
to everyone the vital importance of water as a fundamental part of human life. To support this cause, we invites researchers to contribute
articles that highlight the importance of Water. To encourage participation, IJH is offering a 30% discount on all submissions received on
or before March 22nd.
World Water Day is celebrated globally on March 22nd. Water is the most essential source for sustaining
life, and it plays an important role in the well-being of every human being. From the International Journal of Hydrology, we wish to convey
to everyone the vital importance of water as a fundamental part of human life. To support this cause, we invites researchers to contribute
articles that highlight the importance of Water. To encourage participation, IJH is offering a 30% discount on all submissions received on
or before March 22nd.