International Journal of
eISSN: 2577-8269


Mini Review Volume 1 Issue 4
1Major General Hugh G Robinson Center for Neuropsychiatry Studies, USA
2Faculty of Arts and Sciences, Harvard University, USA
3Department of Family and Community Medicine, Western Michigan University, USA
Correspondence: Tony L Brown, Faculty of Arts and Sciences, Harvard University, USA
Received: August 08, 2017 | Published: November 24, 2017
Citation: Stashauna K, David N, Suzette H, et al. The correlation between diabetes mellitus type ii and the increased risk of alzheimer’s disease: a collaborative treatment approach. Int J Fam Commun Med. 2017;1(4):68-72. DOI: 10.15406/ijfcm.2017.01.00019
Diabetes Mellitus type II is among the most common and debilitating diseases in the United States. Each year, the incidence of diabetes in the U.S. is 1.4 million.1 It is estimated that by the year 2050, if current trends persists that one-third of American adult population will be diagnosed with diabetes.1 Statistics have indicated that Alzheimer’s disease affects approximately one-ninth (11%) of older Americans, ages 65 and older.2 It is further established that there will be a steady annual increase in the incidence and prevalence of AD, as older Americans continue to live longer than 65 years.4 This paper supports the literature that links diabetes with Alzheimer’s disease. It recognizes that the coexisting complications of diabetes mellitus type II is both pervasive and disheartening. Nonetheless, we have identified both physiological and pharmacological factors that are relevant to the treatment of this epidemic. In addition, we contend that further research is needed to prevent and effectively treat both diseases. An expected outcome is that sustained circumvention of the negative side effects could lead to enhanced quality of life. We conclude that based on gender and racial disparities, the most at risk populations must be identified and targeted by healthcare educators and public health officials.3 One implication is that responsible intervention will require a collaborative approach, which combines lifestyle changes and conventional medical approaches. This can result in improved clinical outcomes, patient longevity, and overall enhanced quality of life.
CI: Cognitive Impairments; AD: Alzheimer’s Disease; HDLs: High-Density Lipoproteins; VLDLs: Very-Low-Density Lipoproteins; IDLs: Intermediate-Density Lipoproteins; IAPP: Islet Amyloid Polypeptide
Diabetes Mellitus type II is among the most common and debilitating diseases in the United States. There is evidence to support a strong association between type II Diabetes and AD, in addition to the relationship of APO E with both diseases. This is indicated in mounting evidence now suggesting that there exists a correlation between diabetes mellitus type II and Alzheimer’s disease (AD).5-31 Diabetes type II individuals produce higher amounts of insulin compared to non-diabetics. Physiologically, the release and function of Insulin is a tightly regulated process, which enables the body to balance its metabolic needs. This hormone possesses the ability to cross the blood brain barrier and interrupt brain chemistry. When this happens, optimal brain function diminishes leading to mild cognitive impairments (CI).8,9 It has been described that reduced insulin action or insulin deficiency could contribute to cognitive deficiencies.30-33 Beta-amyloids (Aβ), are proteins unique to both Diabetes and Alzheimer’s disease. Ultimately, multiple amyloid proteins build up in the brain because of excess cerebral insulin leading to Alzheimer’s disease. Although Aβ expression has been detected in extrabrain regions, its effects on these peripheral tissues are not yet fully understood. Studies in rodents suggest a role for Aβ in regulating the ability of peripheral tissues to respond to insulin.8-37
Given that diabetes mellitus type II and AD are complex diseases, it is evident that there are several contributing factors that may increase one’s likelihood of developing this disease.10 In 2014, Alzheimer’s disease was recorded as the fifth leading cause of death in the U.S. and Diabetes Mellitus was ranked the sixth leading cause of death.11 The number of Alzheimer’s disease related deaths totaled 93, 541 and diabetes mellitus related deaths reached a staggering total of 76,488.1-4 Of particular note is the fact that the incidence of diabetes is rapidly rising amongst individuals less than twenty years of age. According to this statistic approximately 208,000 American youths are diagnosed with diabetes.1 These findings are difficult to ignore and compel healthcare professionals to implement strategies that will support healthy lifestyle changes. This approach can decrease the incidence of both diseases while improving overall patient outcomes and preventing complications to those already diagnosed.
Diabetes and American society
According to the American Diabetes Association, the prevalence of diabetes in 2012 was 29.1 million Americans or 9.3% of the population. Among the 29.1 million, 21 million were diagnosed and 8.1 million were never diagnosed.1 The prevalence in seniors, age 65 and older, was 11.8 million or 25.9% both who were diagnosed and undiagnosed.1 Each year, the incidence of diabetes in the U.S. is 1.4 million. It is estimated that by the year 2050, should current trends persists, one-third of American adult population will be diagnosed with diabetes.1 There is evidence to support the notion that racial and ethnic disparities exist among those with diabetes and Alzheimer’s disease.1 In Figure 1, statistics have shown that 15.9% of American Indians, 13.2% non-Hispanic blacks and 12.8% Hispanics are currently diagnosed with diabetes. In addition the reported numbers of infected individuals are decreased to 9.0% of Asian Americans and 7.6% in non-Hispanic white Americans. The high incidence of diabetes type II among these groups can be attributed to the following predisposing factors; 1) increased accessibility to high calorie refined foods, 2) obesity, and 3) a sedentary lifestyle.8
A study was conducted to determine how geographical location and dietary and life style practices can impact the incidence and prevalence of diabetes. The findings showed that people living in densely populated areas are at a higher risk for diabetes when compared to those who living in rural areas .5 The diagnosis and treatment of diabetes have far reaching financial implications that must also be addressed. In 2012 healthcare expenditure on total costs of diagnosed diabetes was an alarming $245 billion. Another $176 billion were spent on direct medical costs and $69 billion was lost on reduced productivity related to complications of this disease.1
Studies have indicated that Alzheimer’s disease affects approximately 15% of older Americans, between ages 65 and 74, (Figure 2).3 Also demonstrated in figure 2, 38% of Americans, age 85 and older are affected by Alzheimer’s disease. It is further established, (Figure 3) that there will be a steady annual increase in incidence and prevalence of AD, as older Americans continue to live longer than 65 years.2-5 The prevalence in seniors, ages 65 and older were a staggering 5.1 million in 2015. A study using the data from the U.S. Census and the Chicago Health and Aging Project revealed a higher prevalence in women.6,7 Approximately two-thirds of individuals with Alzheimer’s disease are women; 3.2 million of the 5.1 million individuals living with this disease are women and 1.9 million are men.4

Figure 2 Data from Gaugler et al.2
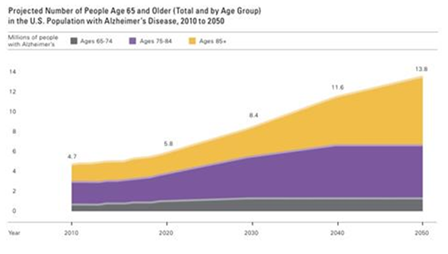
Figure 3 Data from Gaugler et al.2
There is data to support that major risk factors for susceptibility to Alzheimer’s disease are an individual’s age and gender.14 In (Figure 4), The Framingham Study inferred that more women than men have the disease. This is because more women live longer than men. Men are more prone to cardiovascular problems compared to women of middle age.2-12 As for the men living longer than age 65, they usually have a lower risk for cardiovascular disease based on their health history and hence, a lower risk for dementia.2-13 Since having a higher level of education decreased the likelihood of developing AD, males had a lesser chance of developing AD. This is owing to the fact that men age 65 and older had a higher educational status than women born in the early1900s. 14Fewer women acquired higher education in that era and were unlikely to achieve a higher level on education.14 Based upon the study’s findings, this disparity in prevalence seem to suggest that higher education and brain stimulation decreases the risk of AD.
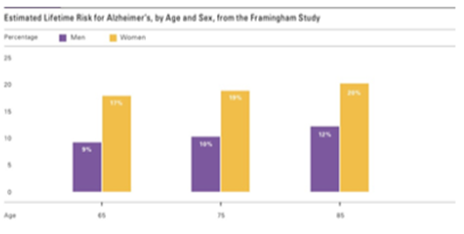
Figure 4 Data from Gaugler et al.2
Linking diabetes and AD
Several studies have shown that the gene ApoE (Apolipoprotein), specifically the allele E4 (ε4), increases one’s risk for developing Alzheimer’s disease. This is considered one of the strongest indicators for the detection of AD.6-13 ApoE is among a group of proteins found in some lipoproteins; for example, Chylomicrons, High-Density lipoproteins (HDLs), Intermediate-Density Lipoproteins (IDLs), and Very-Low-Density lipoproteins (VLDLs).15 ApoE is vital for the transport of lipids throughout the body. Some research data allow that there are noticeable gender biases with respects to the gene ApoE (ε4).2-13 It is interesting to note that there is a higher probability in women diagnosed with Alzheimer’s disease compared to their male counterparts.13 This is partly a result of the increased amounts of ApoE (ε4) in females due to estrogen and the pathology of tau protein.2-17 Individuals with (CI), usually have higher levels of fasting insulin. This finding is supportive of the belief that there exists an interrelationship between insulin resistance and AD.32 In sum, individuals with higher fasting insulin are more prone to develop AD.7-13
The combination of allele ε4 and glucose intolerance increases the risk factors for AD. However, ε4 levels did not affect fasting glucose values but it did affect glucose tolerance.7-13 In the same studies men had higher fasting glucose than women but women had higher levels of ε4. This is significant because ApoE ε4 considerably altered the risk for AD in individuals with diabetes. In addition, mutations in the islet amyloid polypeptide (IAPP) gene have been linked to an increased risk for type II Diabetes.38 Furthermore, the combination of diabetes and the APOE ε4 gene exacerbated the predisposing risk factors of AD.6-14
Complications of diabetes type II and AD
As the prevalence of diabetes increases, so too will the incidence of AD increase; this is because of the interplay of these two diseases. The coexisting complications of diabetes mellitus type II are both pervasive and disheartening. Some of the most common complications linked to diabetes mellitus are kidney failure, ocular impairment, CI, decreased blood flow to the brain, stroke, and cardiovascular complications.16-23 These complications are linked to insulin resistance with abnormalities in lipoproteins such as high levels of low-density lipoproteins, low levels of high-density lipoproteins, and high levels of triglycerides.10 Fasting hyperglycemia is the result of insulin resistance. This is predicated upon the failure to suppress hepatic gluconeogenesis. Insulin resistance is a result of a dense calorie diet, a sedentary lifestyle, and the usage of some medications like steroids.6 When fasting glucose is above the normal range the body is unable to compensate for this and insulin becomes inadequate to return sugar levels to normal. Also, insulin resistance leads to an increase in IAPP production, since IAPP and insulin are cosecreted and subjected to the same regulatory mechanisms.39 Thus, in the prediabetes stages, obesity and insulin resistance may increase IAPP production.
Several complications associated with Alzheimer’s disease include brain shrinkage, memory loss, loss of inhibitions, depression, incontinence, aphasia, dysphagia and aspiration. There are proteins called beta-amyloids that are found in the lipid membranes of nerve cells, which destroy brain cells and interfere with inter-cellular communication. When these proteins aggregate they are called plaques.24 A very important protein called tau is most abundantly located in neurons. Their primary role is to stabilize microtubules in order to properly transport nutrients throughout these microtubules.15 When they are unable to play this role, they become tangled and ineffective in transporting nutrients. Due to this ineffective transport system resulting from these twists, brain cells die from lack of vital nutrients and subsequently leading to the aforementioned complications. These are the same plaques and tangles that can be seen in the brain tissues affected with Alzheimer disease.17-24
Therapeutic interventions and mechanism of action of drugs
There are several common interventions known to healthcare professionals that are effective in treating diabetes mellitus type II disease such as lifestyle changes (e.g. increased physical activity and consuming a healthy diet) and pharmacological treatments. Diabetes therapies that enhance insulin secretion, sensitivity, or signaling activity, such as metformin, peroxisome proliferator-activated receptor g agonists, and GLP-1, have been shown to prevent neurodegeneration in model systems.40-42 Furthermore, clinical trials are underway to assess the benefits of intranasal insulin (NCT01767909) and diabetes drugs such as liraglutide (NCT01843075) for the treatment of AD. The mechanism of action of metformin depends on the alterations in cellular energy metabolism (the increased AMP/ATP ratio).25 Metformin lowers glucose by inhibiting hepatic gluconeogenesis and opposing the action of glucagon. Metformin-mediated inhibition of mitochondrial complex I results in defective cAMP and protein kinase A regulation of glycogen, lipid metabolism, and sugar.25
The prevention of AD also includes lifestyle changes similar to those of diabetes (increased brain activity, higher education, physical activity, and consuming a healthy diet). Pharmacological treatments for AD are categorized as cholinesterase inhibitors like Aricept, Razadyne, and Exelon, and memantine like Namenda. The mechanism of action of Cholinesterase inhibitors is to impede the process that breaks down acetylcholine, which is a crucial neurotransmitter.26 Memantine is an NMDA (N-methyl-D-aspartate) receptor antagonist that regulates the activity of glutamate, another vital neurotransmitter in the brain, which is crucial in learning and retention. Attachment of glutamate to cell surface called NMDA receptors permits calcium to enter the cell. This process is important for cell signaling, as well as learning and memory. However, in Alzheimer’s disease, excess glutamate can be released from injured cells, resulting in continued exposure to calcium. This prolonged exposure to calcium speeds up cellular destruction. Memantine aids in the prevention of this destruction by partially blocking the NMDA receptors.26
Collaborative treatment modalities
There are several forms of non-pharmacological modalities that can both prevent and treat diabetes mellitus type II and AD. With convincing evidence that diabetes type II can be prevented or delayed, strategies to implement the primary prevention of this disease in high-risk subjects are urgently needed. A randomized trial revealed that the dietary intervention that is necessary for the prevention and treatment of diabetes type II consists of decreased intake of refined sugars, saturated fats, simple carbohydrates, and alcohol and an increase intake of complex carbohydrates and high fiber intake like vegetables.27,28 These studies suggest that there is a better chance of decreasing the risk of diabetes type II with consistent healthy lifestyles changes as opposed to pharmacological interventions.43-47 One study concluded that some of the most effective forms of treatments for AD are enriched group cognitive training or physical exercise and music, and cranial or dorsal stimulation.29 This method of treatment may prove to be cost-effective with improved quality of life for both the caregiver and the individual with AD.29 Pharmacotherapies appear to delay the progress of AD. The current limits on the efficacy of medications and the requirement for an array of alternative treatments emphasize the need for more pertinent evaluations of non-pharmacological interventions in AD.43-47 Since the incidence and prevalence of diabetes co-infection with AD are highest in socioeconomically challenged Blacks and Hispanics, measures at primary prevention must be developed to target these specific populations.1-3
Despite the surplus of research conducted on both diabetes mellitus type II and Alzheimer’s disease, further research is needed to prevent and effectively treat both diseases. This allows for sustained circumvention of the debilitating effects and can lead to enhanced quality of life. In light of gender and racial disparities, the most at risk populations must be identified and targeted by healthcare educators and public health officials. These individuals must be willing to develop effective educational and clinical tools leading to increased awareness and subsequent disease prevention. Although healthcare professionals and researchers are the main individuals to implement the gold standard of effective methodologies for optimal clinical outcomes, pharmacological treatments have been their preferred intervention. Given that the effects of these diseases are multifaceted; any responsible intervention requires a collaborative approach. In this way, improved clinical outcomes, patient longevity, and overall quality of life can be realized.
None.
The author declares no conflict of interest.

©2017 Stashauna, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.