International Journal of
eISSN: 2577-8269


Research Article Volume 6 Issue 3
Clinical Chemistry Department, Hospital Universitari i Politècnic La Fe, Valencia, Spain
Correspondence: Óscar Fuster, Clinical Chemistry Department, Hospital Universitari i Politècnic La Fe Avenida Fernando Abril Martorell 106, 46026 Valencia, Spain, Tel +3496961244808
Received: April 18, 2022 | Published: May 17, 2022
Citation: Tofan L, Piqueras M, Fuster O, et al. Monocytopenia in hairy cell leukemia, a difficult feature to detect using sysmex XN series hematology analyzer. Int J Fam Commun Med. 2022;6(3):94-97. DOI: 10.15406/ijfcm.2022.06.00271
Hairy cell leukemia (HCL) is a relatively rare chronic B-cell malignancy that involves the bone marrow, spleen and peripheral blood. Monocytopenia could represent a clue for the suspicion of HCL with complete blood counts (CBCs) and careful assessment of the cell morphology being the first steps in the identification of hairy cells. The purpose of our study is to describe our experience with cell count and flag performance provided by the XN-module in a continuous series of six HCL patients diagnosed in the last two years. The final diagnosis was made by immunophenotypic and genetic analysis. Five out of six patients presented relative monocytosis on automated differential count. Nevertheless, the relative monocyte count was overestimated by the analyzer regarding to the manual count in all cases. The smear revision showed that most cells classified as monocytes were primarily hairy cells which afterwards were confirmed by the immunophenotype. All patients showed potentially pathologic WDF scatergrams or flags and were selected for microscopic smear review. In five of the six patients the WDF channel displayed the “Blasts/Abn Lympho?” flag and triggered the reflex reanalysis using the WPC channel. All samples presented lack or abnormal position of the monocytes cluster in the WPC scattergram. As a conclusion the monocytopenia should be taken with caution for the initial screening of hairy cell leukemia. Instead, an abnormal appearance of WDF scattergram and the lack or an ectopic position of the monocyte cluster in WPC scattergram should be considered to initiate the review of the peripheral blood smear.
Hairy cell leukemia (HCL) is a relatively rare chronic B-cell malignancy that involves the bone marrow, spleen, and peripheral blood. Monocytopenia could represent a clue for the suspicion of HCL. Complete blood counts (CBCs) and careful assessment of the cell morphology are the first steps in the identification of hairy cells. Nevertheless, the final diagnosis of HCL is based on immunophenotypic and genetic analysis. Currently, the initial screening for blood pathologies is based on cell count as well as flagging performance and cell population classification into well-defined clusters using automated hematology analyzers. This is the case of Sysmex XN, a hematology analyzer based on fluorescent staining and laser scatter technology, which provides various channels for white blood cell (WBC) count and determination of WBC populations: white cell nucleated (WNR) channel, white cell differential (WDF) channel and white cell precursor (WPC) channel with a visual presentation in scattergrams showing an optimized separation of the cell populations.1 The incorporation of new parameters and systems of flagging offers a useful tool to guide towards a rapid diagnostic when morphologic or quantitative hematologic alterations are present. However, morphological alterations presented in pathological cells sometimes prevent the complete and correct separation of cell populations in the differential scattergram, which leads to an erroneous automatic count that can cloak diagnostic criteria or analytical characteristics. This is a recently described finding in HCL when using analyzers based on physical properties technology.2 Furthermore, the suspicion based in a single patient that analyzers using fluorescent staining and laser scatter technology (Sysmex, Kobe, Japan) do not also properly classify HCL must be confirmed.3 In this report we describe our experience with cell count and flags provided by the XN-module in a continuous series of HCL patients.
The study included 6 patients with HCL diagnosed in the last two years. The samples were analyzed using the Sysmex XN hematology analyzer. The automatic results were compared with the duplicate cell count performed by two expert observers in the blood smear. Due to the retrospective character of the study, the 2 expert observers were aware of both the diagnosis of the patients and the differential count and the alarms flags emitted by the analyzer. The final diagnosis was made by immunophenotypic and genetic analysis following the World Health Organization Consensus Guideline. The study was carried out in accordance with the Declaration of Helsinki in line with any relevant local legislation.
Among the 6 patients with HCL, five were males and one female with a mean of 58±21 years old. The total and differential WBC count of the patients as well the hemoglobin concentration and platelets cell count are shown in Table 1. Three patients presented normal value of WBC, two presented leukocytosis and one patient had moderate leukopenia. Five out of six patients presented relative monocytosis on automated differential count. Nevertheless the relative monocyte count was overestimated by the analyzer compared to the manual count in all cases. The smear revision showed that most cells classified as monocytes were primarily hairy cells which afterwards were confirmed by the immunophenotype (Table 1).
Patient |
Parameter |
Units |
Automated results |
Manual results |
Reference values |
Hemoglobin |
(g/L) |
140 |
130-175 |
||
White Blood Cells |
(x109/L) |
16.6 |
- |
3.5-10.5 |
|
Neutrophils |
(%) |
34.9 |
36 |
35-80 |
|
Lymphocytes |
(%) |
31 |
24 |
20-45 |
|
1 |
Monocytes |
(%) |
29.1 |
4 |
12-Apr |
Eosinophils |
(%) |
4.3 |
4 |
5-Jan |
|
Basophils |
(%) |
0.7 |
1 |
0-1.5 |
|
Hairy Cells |
(%) |
- |
31 |
||
Platelets |
(x109/L) |
183 |
150-400 |
||
Hemoglobin |
(g/L) |
104 |
130-175 |
||
White Blood Cells |
(x109/L) |
11.88 |
- |
3.5-10.5 |
|
Neutrophils |
(%) |
11.1 |
19 |
35-80 |
|
Lymphocytes |
(%) |
24.1 |
27 |
20-45 |
|
2 |
Monocytes |
(%) |
63.8 |
3 |
12-Apr |
Eosinophils |
(%) |
0.8 |
2 |
5-Jan |
|
Basophils |
(%) |
0.2 |
0 |
0-1.5 |
|
Hairy Cells |
(%) |
- |
49 |
||
Platelets |
(x109/L) |
35 |
150-400 |
||
Hemoglobin |
(g/L) |
9.9 |
130-175 |
||
White Blood Cells |
(x109/L) |
8.26 |
- |
3.5-10.5 |
|
Neutrophils |
(%) |
39.1 |
45 |
35-80 |
|
Lymphocytes |
(%) |
33.1 |
23 |
20-45 |
|
3 |
Monocytes |
(%) |
25.8 |
6 |
12-Apr |
Eosinophils |
(%) |
1.5 |
1 |
5-Jan |
|
Basophils |
(%) |
0.5 |
3 |
0-1.5 |
|
Hairy Cells |
(%) |
- |
22 |
||
Platelets |
(x109/L) |
163 |
150-400 |
||
Hemoglobin |
(g/L) |
133 |
130-175 |
||
White Blood Cells |
(x109/L) |
7.15 |
- |
3.5-10.5 |
|
Neutrophils |
(%) |
23.3 |
25 |
35-80 |
|
Lymphocytes |
(%) |
44.3 |
30 |
20-45 |
|
4 |
Monocytes |
(%) |
32 |
2 |
12-Apr |
Eosinophils |
(%) |
0.3 |
1 |
5-Jan |
|
Basophils |
(%) |
0.1 |
0 |
0-1.5 |
|
Hairy Cells |
(%) |
- |
32 |
||
Platelets |
(x109/L) |
54 |
150-400 |
||
Hemoglobin |
(g/L) |
85 |
130-175 |
||
White Blood Cells |
(x109/L) |
5.9 |
- |
3.5-10.5 |
|
Neutrophils |
(%) |
13.2 |
15 |
35-80 |
|
Lymphocytes |
(%) |
34.4 |
30 |
20-45 |
|
5 |
Monocytes |
(%) |
52.2 |
0 |
12-Apr |
Eosinophils |
(%) |
0.2 |
1 |
5-Jan |
|
Basophils |
(%) |
0 |
0 |
0-1.5 |
|
Hairy Cells |
(%) |
- |
54 |
||
Platelets |
(x109/L) |
56 |
150-400 |
||
Hemoglobin |
(g/L) |
121 |
130-175 |
||
White Blood Cells |
(x109/L) |
1.67 |
- |
3.5-10.5 |
|
Neutrophils |
(%) |
31.1 |
51 |
35-80 |
|
Lymphocytes |
(%) |
59.9 |
38 |
20-45 |
|
6 |
Monocytes |
(%) |
6 |
1 |
12-Apr |
Eosinophils |
(%) |
2.4 |
0 |
5-Jan |
|
Basophils |
(%) |
0.6 |
0 |
0-1.5 |
|
Hairy Cells |
(%) |
- |
10 |
||
Platelets |
(x109/L) |
119 |
150-400 |
Table 1 Cell blood count and differential comparison between automated and manual results in hairy cell leukemia patients
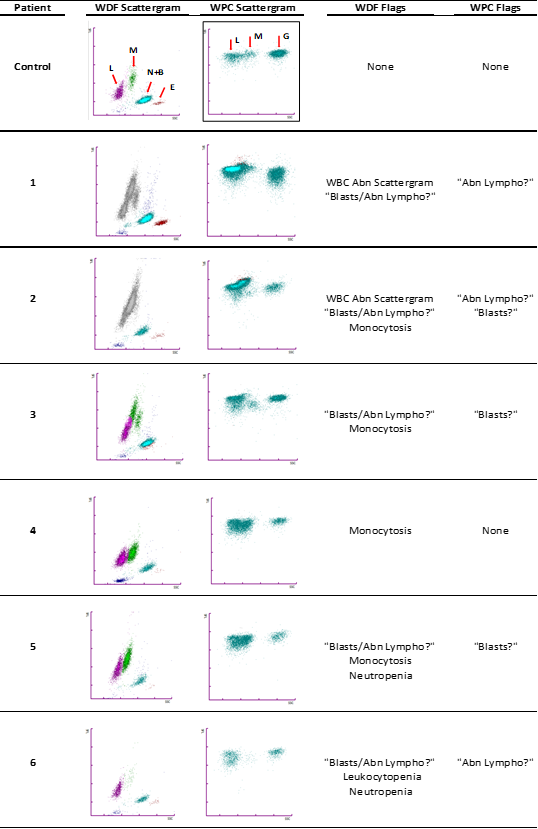
Figure 1 Scattergram and flags in our six patients with hairy cell leukemia vs normal control. (WDF= white cell differential; WPC= white cell precursor channel; L=lymphocytes; M= monocytes; N= neutrophils; B= basophils; E= eosinophils; G= granulocytes).
Regarding the flags, in our case the flagging process was able to point all six patients as potentially pathologic and select them for a microscopic smear review (Figure 1). In five of the six patients, including the patient with leukopenia, the WDF channel of the analyzer flagged the risk of malignant cells presence by generating the “Blasts/Abn Lympho?” flag and triggered the reflex reanalysis using the WPC channel. The remaining patient was selected for smear review due to the aspect of its scattergram with overlapping clusters of lymphocytes and monocytes and the numerical criteria of monocytosis established by our laboratory, with a cut of point of 1.3x109/L (patient 4, Figure 1).
The WPC channel offers the possibility of more specific flags after triggering in the WDF channel the “Blasts/Abn Lympho?” flag. It can remove the flag or classify the cells into blasts (“Blasts?” flag) or neoplastic cells (“Abn Lympho?” flag) with certain degree of differentiation based on morphological and biochemical properties of the reagent and the cells.4 According to the manufacturer, initially, surfactants in Lysercell WPC produce red blood cells and platelets lysis and alter the lipid structure of white blood cells membrane perforating it. This step enables the entrance of fluorescence marker into the leukocyte up to a point based on its content of membrane phospholipids. Immature cells such as blast present a lower content of lipid than differentiated cells and consequently one of the lowest fluorescence signals of all cells. Finally, the different subpopulations are separated on the basis of cell size or forward scatter, cell complexity or side scatter and fluorescence signal.
In our series WPC channel classified two samples with “Abn Lympho?”, two samples with “Blasts?” and one sample with both flags. All samples presented abnormal WPC scattergram: the majority presented lack of the monocytes cluster (patients 1,2,4,5 and 6 (Figure 1). The remaining patient showed a monocyte cluster probably related to its normal monocyte count but with an abnormal position lower than control samples (patient 3, Figure 1).
Monocytopenia is a relatively sensitive and specific finding of HCL5 that could guide the clinician to an initial diagnostic assessment of HCL. However, in clinical practice this consistent feature could be difficult to detect by the autoanalyzers which is a challenge for the laboratory diagnosis. The WDF channel of XN series provides an optimized separation of the mononuclear cells compared to previous models.6 Even so, the morphologic changes of the leukemic cells with its bigger size than normal lymphocytes and wider and irregular cytoplasm produce an impaired separation and an inaccurate count of lymphocytes and monocytes. However, our data suggest that cell cluster analysis obtained by WPC channel could improve the initial analytical evaluation performed by WDF by offering a visual clue of the presence of monocytopenia. The complete absence of monocyte cluster or its abnormal position in the presence of monocytosis should raise the suspicion of an impaired cell blood count that demands a blood smear review. Our results confirm the similar findings described in a previous study concerning the performance of XN-series that included a single HCL patient with abnormal appearance of the monocyte´s area in WPC scattergram.3s However, we also found differences regarding the findings highlighted by Seghezzi et al.3 in the WDF scattergram between one sample from a HCL patient vs other sample from a splenic marginal zone lymphoma (SMZL) patient, both cases with the same low count of villous cells (3%). The authors describe the WDF scattergram of the first case with an abnormal monocyte cluster clearly lower than the usual position and the second one with an abnormal lymphocyte double cluster. However, our experience with more HCL cases and higher percentage of circulating villous lymphocytes showed that both scattergrams types can be present in HCL patients. We hypothesized that despite BRAF-V600E mutation is now considered as the molecular hallmark of the disease, the emergence of new mutations and specifically, alterations of the cell cycle8 could condition the heterogeneity of the analytical presentation, with wide-ranging morphology and therefore different locations of the cell populations in the scattergrams.
The WDF flagging performance was more consistent displaying “Blasts/Abn Lympho?” flag in our series of cases than in previous reports.7 However the mean of circulating hairy cells in peripheral blood was higher (19.6%) in our group of patients than in the previously cited (9.4%). Nevertheless, the more specific WPC channel, showed more uneven results when classifying pathological cells as “Abn Lympho?” and it was not able to improve the specificity of the WDF.
In conclusion, up until the count of the different cell populations and the specificity of the WPC channel are improved, the monocytopenia feature and the “Abn Lympho?” flag should be taken with caution for the initial screening of hairy cell leukemia. Instead, the abnormal classification of cells in the WDF scattergram and its “Blasts/Abn Lympho?” flag as well as the lack or abnormal position of monocyte cluster in WPC scattergram should be considered to initiate the review of the peripheral blood smear. The analyzer must optimize its separation between lymphocytes and monocytes in HCL in order to detect monocytopenia and the presence of leukemic cells in blood.
None.

©2022 Tofan, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.