International Journal of
eISSN: 2577-8269


Case Report Volume 7 Issue 3
1Rheumatology, Instituto Hospital de Base, ESCS, Brazil
2Otolaryngology, Instituto Hospital de Base, Brazil, ESCS, Brazil
3Pathology, Instituto Hospital de Base, Brazil, ESCS, Brazil
Correspondence: Luciana Nunes Assis Daameche, Department of Rheumatology, Rheumatology resident by ESCS at instituto hospital de base, SMHS, Área Especial, Quadra 101, Asa Sul, Brasília (DF), CEP 70.330-150, Tel (61) 3550-8900
Received: May 22, 2023 | Published: June 6, 2023
Citation: Daameche LNA, Lins CE, Pires TO, et al. Leprosy mimicking ANCA-associated vasculitis. Int J Fam Commun Med. 2023;7(3):83-86. DOI: 10.15406/ijfcm.2023.07.00317
Vasculitis represents a large group of diseases, classified between primary and secondary. Diagnosis of primary vasculitis is a challenge in medical practice, since there are wide and heterogeneous clinical manifestations, with diagnostic criteria still scarce. Some clinical manifestations are common: constitutional status, myalgia, arthralgia, arthritis, papules, nodules and ulcers. One of the clinical forms is the limited one in which patients with upper respiratory tract involvement often evolve with systemic disease. Most patients have nasal, sinus or ear involvement that may be present weeks or months before other symptoms. Secondary vasculitis can be related to infections, drugs, toxic substances and neoplasms. The virchowian and dimorphic form of leprosy has similar clinical and serological characteristics with rheumatological diseases. As it is an endemic disease in Brazil, there is a description of a wide variety of clinical presentations, so making a differential diagnosis is essential.
Vasculitis represents a large group of diseases, classified between primary and secondary. Secondary vasculitis are the most frequent, capable of mimicking primary autoimmune vasculitis, and are also identified as vasculitis -like, look-alike vasculitis and mimic vasculitis syndromes. Extensive anamnesis and adequate complementary exams should be carried out, as it is essential to consider differential diagnoses. If you perform a wrong diagnosis, the therapies are potentially harmful, can delay the diagnosis and sometimes be fatal.1
Diagnosis of primary vasculitis is a challenge in medical practice, since there are broad and heterogeneous clinical manifestations, with diagnostic criteria still scarce. The increase in knowledge about this condition has made it possible to reduce the delay in diagnosis with the use of ANCA serology (antibody against the cytoplasm of neutrophils), and has increased the incidence and age of patients at diagnosis, which demonstrates underdiagnosis.2 ANCA can be divided into anti-myeloperoxidase (MPO) antibodies, which show a perinuclear fluorescent pattern (p-ANCA) and anti-proteinase 3 (PR3) with a cytoplasmic immunofluorescence pattern (c-ANCA). They are associated with necrotizing vasculitis, mainly granulomatous polyangiitis, microscopic polyangiitis and idiopathic crescentic glomerulonephritis.3
ANCA induces inflammation of blood vessels with consequent flow obstruction and ischemic tissue necrosis; secondary to immunopathogenic mechanisms, after antigenic stimuli associated with genetic predisposition and environmental factors. Some clinical manifestations are common: constitutional condition, myalgia, arthralgia, arthritis, purpura, papules, nodules and ulcers. Symptoms that can distinguish between vasculitides are: mononeuritis multiplex, peripheral neuropathy, alveolitis, pulmonary hemorrhage, pulmonary nodules, asthma, sinusitis, abnormal urinary sediment, proteinuria, convulsive crises, visual alterations, among others.4
Secondary vasculitis may be related to infections such as leprosy; drugs such as antibiotics, angiotensin-converting enzymes, penicillamine, propylthiouracil, and cytotoxic drugs; toxic substances and neoplasms.3 The Virchowian and dimorphic form of leprosy has clinical and serological characteristics similar to rheumatological diseases. They may present cutaneous lesions with a variable spectrum of involvement with ulcerations, ischemic necrosis, vesicles, blisters, purpura, nodules and malar rash to systemic involvement such as hepatosplenomegaly, arthralgia and polyarthritis.5
Patients with leprosy may have changes in rheumatoid factor, anti-cardiolipin and ANCA.5 Thus, in a study of 120 patients diagnosed with leprosy (77 of the lepromatous form and 43 of the tuberculoid form), 35% had positive RF and 55.8% ANA. The presence of ANCA was studied in 68 patients with leprosy, finding ANCA-p in 31% of those with the lepromatous form and 16% of those with the borderline form; ANCA-c was seen in 5% of those with the lepromatous form.6 Other less frequent manifestations are Raynaud's phenomenon, livedo reticularis, skin thickening and periorbital edema5. Therefore, it is important to expand the range of differential diagnoses of diseases that can affect blood vessels, as shown in Table 1.
Diseases can affect large vessels |
1- mycotic aneurysms |
2- endocarditis |
3- syphilis |
4- HIV |
Diseases may affect medium-sized vessels |
1- hepatitis B |
2- hepatitis C |
3- HIV |
4- atherosclerosis |
5- lymphoma |
6- leukemia |
7- thrombotic thrombocytopenic purpura |
8- antiphospholipid antibody syndrome |
Small vessel disease |
1- cocaine (they present lesions in the nasal midline) |
2- lymphoproliferative disease1 |
Table 1 Differential diagnoses of ANCA-associated vasculitis ANCA
A 37-year-old female patient was admitted to the Otorhinolaryngology emergency room at Hospital de Base Institute due to acute rhinosinusitis complicated with cellulitis on the face. On examination, he had cellulitis on the nasal dorsum. Rhinoscopy showed pervious nasal cavities with crusts, inferior turbinate hypertrophy and friable mucosa. Intravenous antibiotic therapy with clindamycin and ceftriaxone was started.
Carrier of allergic rhinitis since the age of 17. 3 years ago, he started with ulnar mononeuritis and sensorineural polyneuropathy of the lower limbs. She evolved with polyarthralgia and recurrent rhinosinusitis. After inappropriate use of Azithromycin, she presented papulovesicular lesions on the lower limbs that progressed to ulcerated and crusted lesions (Figure 1, 2 and 3). It was previously investigated on an outpatient basis, but without diagnostic definition. The following were requested: ANCA, ANA, bacilloscopy of the lymph of the earlobes and elbows, electroneuromyography of the lower limbs, magnetic resonance imaging of the skull, ANCA (anti-neutrophil cytoplasmic antibody) and ANA (anti-nuclear factor).
Laboratory tests showed iron deficiency anemia, thrombocytosis and did not close diagnostic criteria for collagenosis (ANA, complement, anti DNA and hemolytic anemia) or viral diseases (hepatitis B and C, HIV and VDRL), as well as negative blood cultures. Requested ANCA and anti proteinase 3 on admission, also negative with increased inflammation tests.
Computed tomography of the facial sinuses showed mucous thickening of the maxillary, ethmoid and frontal sinuses on the left, bilateral sphenoid sinuses suggestive of sinusopathy. It also showed obliteration of the left ostiomeatal complex and mucous thickening of the left nasal concha (Figure 4, 5). There was no associated renal or pulmonary alteration. Electroneuromyography of the limbs during this hospitalization showed asymmetrical, axonal, sensory-motor polyneuropathy, with evidence of active denervation in more distal muscles, which could also represent a picture of mononeuritis multiplex in an advanced stage.
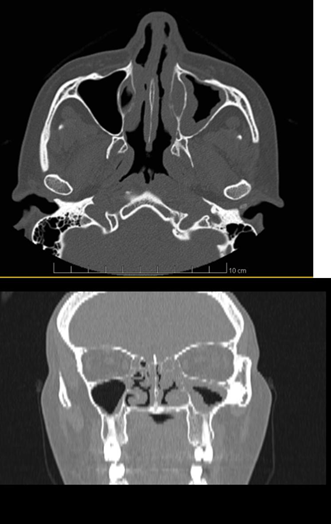
Figure 4,5 Non-contrast-enhanced sinuses computed tomography in axial (Figure 4) and coronal sections (Figure 5) shows mucosal edema in the inferior and middle turbinate of the left nasal fossa, mucosal edema in the inferior turbinate of the right nasal fossa, sinus mucosal thickening left jaw.
In nasal videoendoscopy, pervious nasal cavities were visualized, but with crusts. Ulcerated crusted lesion in the anterior nasal septum on the left and in the vertical portion of the left middle turbinate, crusted lesion on the anterior inferior concha on the right, free cavum. Associated with friable mucosa, especially in the septum region with prominent vascularity (Figure 6, 7). Skin and nasal ulcer biopsies were taken. Anatomopathological report of the nasal ulcer led to the diagnosis of Virchowian Leprosy (Figure 7, 8). The patient was also treated with intravenous iron replacement and prednisone 60 mg/day. She showed significant clinical and laboratory improvement. She was referred to the leprosy outpatient clinic at the University Hospital of Brasília for follow-up and treatment with rifampicin, clofazimine and dapsone.
The main differential diagnosis of the presented case report is granulomatosis with polyangiitis. It is a systemic vasculitis, in which it presents constitutional symptoms, necrotizing granulomatous inflammation mainly of the upper and lower respiratory tract, vasculitis and necrotizing glomerulonephritis that can present positive ANCA.7 One of the clinical forms is the limited one, in which patients with upper respiratory tract involvement often develop a systemic disease, a confounding factor for the diagnosis of the patient in question. Most patients have nasal, sinus or ear involvement that may be present weeks or months before the other symptoms. Perforation of the nasal septum is one of the possible complications of chronic rhinosinusitis and adjacent structures can be affected, causing ocular proptosis and oral ulcers. Respiratory tract manifestations include alveolar hemorrhage with hemoptysis, dyspnea and pleuritic pain, tracheomalacia, subglottic stenosis, pulmonary nodules, interstitial lung diseases are also common.7
About half of the patients have cutaneous manifestations that include urticaria, livedo reticularis, nodules, erythema nodosum, pyoderma gangrenosum and the most common is leukocytoclastic angiitis, which can cause purpura of the lower limbs with focal necrosis and ulcerations. Alterations of the peripheral nervous system occur in 15% of patients. These factors enabled the diagnostic hypothesis of the patient in the aforementioned case report. In order to make the diagnosis, a biopsy is necessary together with an extensive clinical history, a well-executed physical examination and complementary tests. Positive ANCA is present in 82-94% of patients and strongly suggests the diagnosis, but it is not pathognomonic and the investigation should continue. It is common to have leukocytosis, thrombocytosis, elevation of inflammatory tests, anemia, as shown in the reported case.8
Other diagnostic tests include anti-basement membrane, complement, cryoglobulins, hepatitis and HIV serologies, cultures and tuberculosis screening. Chest and sinus CT scans are important to assess whether there are respiratory tract involvements and to assess the best location to perform the biopsy, if possible. Nasal biopsy is limited by presenting false negative or nonspecific results, but it can be useful for differential diagnosis such as infection, malignancy or trauma; diagnostic defining conduct for the reported case.8
Leprosy is an infectious disease caused by Mycobacterium leprae and Mycobacterium lepromatosis. Chronic granulomatous disease10 with high contagion power and low infectivity, currently with effective treatment there is low morbidity, but if the diagnosis and treatment is late it leads to permanent changes. Prevalence is variable; in the US, in 2010, 205 new cases were detected, most of them among immigrants. In 16 countries, more than 1000 cases were registered annually in 2009, with the highest numbers of cases reported in India, Brazil, Indonesia, Bangladesh and Nigeria.9 In which Brazil is the second country in the world with the highest number of absolute cases.10 The transmission mechanism is still not well understood, it is speculated that it is transmitted by the respiratory route, nasal discharge of multibacillary patients and occasionally penetrate through the injured skin. For infection to occur, sufficient exposure and susceptibility are needed: intimate contact with people with multibacillary leprosy, over 30 years old, variants of the NOD2 gene (regulates innate immunity) and immunosuppression.9
As it is an endemic disease in Brazil, there is a description of a wide variety of clinical presentations and, therefore, performing a differential diagnosis is essential.11 The Ridley and Jopling classification of leprosy is based on skin, neurological and biopsy findings that correlate with individual immune capacity. The tuberculoid form, are patients who have a well-developed immunity, have cutaneous lesions with hypopigmented center and hypoesthesia, rarely have bacilli in the tissues. Patients who are unable to contain the infection, due to failure of the cellular immune system, have numerous bacilli and disseminated nodular lesions that configure the Virchowian form. Between these two extremes, there are borderline classes9: borderline-tuberculoid, borderline-virchowian and borderline-borderline.10 The World Health Organization, for therapeutic purposes, defined two forms: paucibacillary (bacilloscopic index less than 2+ or up to 5 cutaneous lesions and/or affected nerve trunk) and multibacillary (bacilloscopic index greater than or equal to 2+ or 5 or more lesions skin and/or more than one nerve trunk affected).10 In addition to nervous and cutaneous involvement, viscera and mucous membranes may be involved. Neurological evaluation should always be performed, considering that its impairment is characteristic of all forms of leprosy and due to the risk of progression with important and irreversible functional alterations.11
Involvement of the nasopharynx will occur in practically all patients with lepromatous leprosy, it may occur in borderline patients and is very rare in indeterminate and tuberculoid cases.12 published for the first time a case of positive bacilloscopy in the nasal mucosa.13 It is known that during the passage of the secretion through the rhinopharynx, contamination of the oral mucosa may occur. However, it is resistant to the appearance of injuries. These occur more frequently in multibacillary patients in the advanced stages of the disease. This suggests that invasion of the oral mucosa is consequent to bacillemia by bacterial dissemination and proliferation. However, in the initial stages, the absence of evident lesions in the oral mucosa does not exclude its involvement. In, Hubscher et al. performed bacilloscopic examinations on clinically normal mucous membranes, which showed bacilli in 7 out of 17 specimens from the tongue, hard palate and gingiva.12
In about 95% of the cases, the nasal mucosa is affected.14 Therefore, it is important to perform an otorhinolaryngological examination for an early diagnosis of leprosy. The involvement has a descending character, starting in the nasal cavities and progressing to the mouth and larynx.12 Initially, crusts formed, nasal congestion and serosanguineous influence by affecting the antero -inferior portion of the nasal septum. As the disease progresses, grayish or pink nodules appear, which are slightly elevated and tend to ulcerate, as a consequence, obstruction of the nasal septum may occur, especially in the cartilaginous portion.15 This continuous process of septum necrosis causes a nose to sink and collapse with an appearance “nose in binoculars”.16
In the Virchowian form, solid, papular, papulo-nodular, nodular lesions, clinical or grouped plaques with symmetrical distribution, thickening of the pinna and madarosis are common. The rhinoscopy performed on the patient should not be ignored, since nasal preservation in these patients is one of the main complaints, especially in the Virchowian form, and may present several alterations, among them11: mucosal infiltration, abnormal dryness, hypertrophy of the nasal conchae, mucous hyperemia, atrophy of the nasal conchae, perforation of the septum and even saddle nose. In the present case report, one patient had nasal involvement with an ulcerated lesion in the middle to left turbinate, right inferior turbinate, friable mucosa and ulceration of the anterior part of the nasal septum.
In the oropharynx, there are papules on the lips and nodules on the tongue, palate and uvula.12,17 In the larynx, the epiglottis and the arytenoepiglottic folds are mainly affected, with granulomatous edema, which, as a consequence, may obstruct the glottic cleft. As a result, the patient will have aphonia and dyspnea.15 In the ears, there is a predominance of involvement of the ear pinnae with thickening, isolated or rosary nodules. Occurs bilaterally in Virchowian and borderline forms. And unilateral, in the indeterminate and tuberculoid form.17
After the explanation above, it is clear the need to carry out differential diagnoses among vasculitis. Primary vasculitis are difficult diseases to diagnose, since diagnostic criteria are scarce, with a wide variety of signs and symptoms, which can evolve with different patterns of involvement and severity. Among the secondary vasculitides, one cannot forget Leprosy, a disease that is still endemic in Brazil, with diverse clinical characteristics that can mimic primary autoimmune vasculitis. Performing a differential diagnosis is fundamental, since late diagnosis or wrong treatment, in some cases, can lead to irreversible sequelae and even be fatal.
None.
The author declares there is no conflict of interest.

©2023 Daameche, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.