International Journal of
eISSN: 2577-8269


Mini Review Volume 2 Issue 1
1Department of Neuropsychiatry, Kagawa University, Japan
2Department of Applied Physics, Waseda University, Japan
3SCA Corporations, Japan
Correspondence: Junichi Danjo, Department of Neuropsychiatry, Kagawa University School of Medicine, Japan, Tel +81-87-898-5111, Fax +81-87-891-2016
Received: November 10, 2017 | Published: January 9, 2018
Citation: Danjo J, Danjo S, Sawada H, et al. Diabetic neuropathy: a focus on the testing method. Int J Fam Commun Med. 2018;2(1):1-5. DOI: 10.15406/ijfcm.2018.02.00027
Purpose of Review: This article describes the classification, diagnosis, examination, mechanism, prevention, and treatment of diabetic neuropathy. This review details a method for examining distal symmetric polyneuropathy (DSPN), which is the most frequent type of diabetic neuropathy.
Recent Findings: We also discuss a new tactile test method that the authors are investigating for the early detection and diagnosis of diabetic neuropathy, which is currently difficult in routine diabetes practice.
Summary: Diabetic neuropathy is a common complication in diabetic patients. However, diabetic neuropathy is not taken into consideration in routine clinical diabetes practice because the examination is complicated and requires an examiner. In the future, inexpensive examination methods that are simple and quantitative and can be performed even by patients are required.
Keywords: Diabetic neuropathy; Asymptomatic diabetic neuropathy; Examination method; Testing method; Tactile test method
DSPN: Distal Symmetric Polyneuropathy; CAN: Cardiovascular Autonomic Neuropathy
Diabetic neuropathy is the most common chronic complication of diabetes. Among the three major complications of diabetes, diabetic neuropathy is frequent and appears earliest after the onset of diabetes. Diabetic neuropathy presents various clinical symptoms from head to toe (and sole). Identification of the early signs of neuropathy in diabetic patients, early diagnosis, and appropriate management in the early stages are very important for the following reasons. First, the early diagnosis of diabetic neuropathy is difficult. Because multiple tests are required for diagnosis, the examination methods are often complicated and time consuming. It is also necessary to rule out differential diagnoses. Non-diabetic neuropathy may occur in patients with diabetes and requires specific treatment. Second, treatments for symptomatic diabetic neuropathy are available. Third, diabetic neuropathy can be asymptomatic in up to 50% of cases. If it is not recognized and preventive foot care has not been implemented, the patient is at risk of injuring the foot. Asymptomatic diabetic neuropathy is a sign of serious complications. In this review, we describe the classification, symptoms, diagnosis, examination, mechanism, prevention, and treatment of diabetic neuropathy. This review details a method for examining distal symmetric polyneuropathy (DSPN), which is the most frequent type of diabetic neuropathy. Additionally, we discuss a new tactile test method that the authors are investigating for the early detection and diagnosis of diabetic neuropathy, which is currently difficult in routine diabetes practice.
(Table 1) The comprehensive Classification of Diabetic Neuropathy.1 Diabetic neuropathy is roughly divided into diffuse neuropathy (sensory neuropathy/autonomic neuropathy) and mononeuropathy. The former is overwhelmingly more frequent and clinically important.
Diabetic Neuropathies |
Diffuse Neuropathy |
DSPN |
Autonomic |
Cardiovascular |
Gastrointestinal |
Urogenital |
Sudomotor dysfunction |
Hypoglycemia unawareness |
Abnormal pupillary function |
Mononeuropathy (Mononeuritis Multiplex) (Atypical Forms) |
Isolated cranial or peripheral nerve (e.g., CN III, ulnar, median, femoral, peroneal) |
Mononeuritis multiplex (if confluent may resemble polyneuropathy) |
Radiculopathy or Polyradiculopathy (Atypical Forms) |
Radiculoplexus neuropathy (a.k.a. lumbosacral polyradiculopathy, proximal motor amyotrophy) |
Thoracic radiculopathy |
Nondiabetic Neuropathies Common in Diabetes |
Pressure palsies |
Chronic inflammatory demyelinating polyneuropathy |
Radiculoplexus neuropathy |
Acute painful small-fiber neuropathies (treatment-induced) |
Table 1 Classification of diabetic neuropathies.1
Diffuse neuropathy
It appears in bilateral symmetry and predominantly involves sensation disorder. The toes and soles are first affected easily. From an early stage, vibration sensation in the lower limbs and the Achilles tendon reflex are reduced. Most cases also exhibit autonomic symptoms.
Mononeuropathy
It is thought that disorders of blood vessels that nourish nerves are deeply involved in the pathology of mononeuropathy, with a sudden onset and frequent symptomatic improvement occurring within 1 year. In the relatively frequently occurring coulometer nerve paralysis, symptoms such as double vision and drooping eyelids, are observed. In addition, diabetic muscle atrophy and the paralysis of cranial nerves (abducent nerve, trochlear nerve, and facial nerve) and limb motor nerves (ulnar nerve and peroneal nerve) can occur.
DSPN
The symptoms of DSPN differ according to the type of sensory fibers involved, as shown below. The most common initial symptoms, including pain and abnormal sensation (burning sensation etc.), occur due to the involvement of small fibers.2-5
Abnormal sensation: Patients may recognize a numb feeling when they are seated for a long time or a feeling of electric shock. Patients often complain of “the sensation of another skin on the sole of the feet” and “a feeling of ants crawling on the feet.”
Pain: Patients describe intense pain such as a “stinging” or “burning” sensation at the tip of the toes The pain frequently increases at night rather than during the day, sometimes leading to sleep disturbances and depression. Hyperalgesia: Patients will recognize pain or an irritating sensation to a greater extent than do healthy people because the nerve fibers related to pain sensation are in a easily excitable state. Although these irritation symptoms are very annoying, they do not necessarily correspond to the degree of progression of diabetic neuropathy; however, they can be improved with glycemic control.
Hypoesthesia (paralysis of sensory nerves): The response to stimuli, such as pain, decreases with the progression of neuropathy. Tactile sensation in the toes decreases, and in serious cases, even major injuries, such as stepping on broken glass or burning with hot water, can occur as pain is not felt instantly.
Autonomic neuropathy
Orthostatic hypotension: When patients stand suddenly, they experience dizziness. This occurs because the mechanism for blood pressure regulation according to the change in posture is impaired.
Sweating abnormality: The sympathetic nerve that regulates sweat gland function is affected in this disorder. A reduced amount of sweating causes skin dryness, resulting in the “cracking” or “chapping” of hands and feet, which is likely to cause infection. Additionally, sweating is an important sign of hypoglycemia; therefore, if sweating is decreased, patients are less likely to be aware of hypoglycemia.
Unaware hypoglycemia: When the blood glucose level falls to ≤70 mg/dL with insulin injection, the biological defense mechanism that promotes the secretion of insulin-antagonist hormones (glucagon and adrenaline) and causes an increase in blood glucose is activated. Moreover, warning symptoms such as sweating, tremors, palpitations, and anxiety, appear, leading to a sympathetic dominant state. However, if the secretion of insulin-antagonist hormones is impaired or the response of the sympathetic nervous system is decreased, there is a danger of severe hypoglycemia occurring without warning symptoms (sudden loss of consciousness and progression to coma). Moreover, when patients lose consciousness while driving a car, the risk of a traffic accident is high and attention to this symptom is necessary.
Gastrointestinal disorders: Gastrointestinal disorders occur when the autonomic nervous system regulating the movement of the gastrointestinal tract is impaired. Symptoms such as depressed movement of food from the stomach to the intestine, slow digestion (stomach asthenia), alternating constipation and diarrhea can occur.
Bladder disorder: The contracting force of the bladder decreases, and urination becomes difficult. In addition, when sensory neuropathy has progressed, patients feel less urinary urgency, and the frequency of urination decreases.
Erectile dysfunction: It occurs in nerve, muscle, and blood vessel disorders related to erection (organic erectile dysfunction), but in some cases, it is psychogenic; hence, differentiation of these etiologies is necessary for treatment.
Mechanism of DSPN
The pathogenesis of DSPN is multifactorial. Among these factors, polyol metabolism enhancement, glycation, protein kinase C activity abnormality, oxidative stress, and metabolic abnormalities seem to be closely involved in the onset and progression of neuropathy, but the detailed mechanism remains unclear.6-9
Because the mechanism has not been sufficiently clarified, fundamental therapeutic drugs have not yet been developed.
Prevention
Prevention is an important factor in the treatment of diabetic neuropathy because no fundamental treatment targeting neuropathy is available. Although the examination method for diabetic neuropathy includes a screening tool that will be described later, patients with early diabetic neuropathy are often unaware; hence, screening for the symptoms and signs of diabetic neuropathy should be performed at the earliest stage of neuropathy. Detection of the stage of neuropathy is important in clinical practice. Preventive methods mainly include the improvement of glycemic control and lifestyle habits. Tight glycemic control leads to the prevention of DSPN.10-15 It has been reported that the strengthening of glycemic control markedly improved the incidence of DSPN by as much as 78% in patients with type 1 diabetes.10-12 Tight glycemic control also leads to the prevention of cardiovascular autonomic neuropathy (CAN) in patients with type 1 diabetes.16 By contrast, glycemic control in type 2 diabetes patients does not consistently reduce the risk of CAN.17 However, improvements in lifestyle habits related to glucose levels and cardiovascular disease risk factors have focused on exercise18,19 or a combination of diet modification and exercise,20 which have been reported to reduce the risk of CAN.
Treatment
The basics of treating diabetic neuropathy involve firm long-term control of blood glucose as well as other complications. In addition, symptoms may be alleviated by using a therapeutic agent according to the disease mechanism. Several pathogenetic pharmacotherapies have been investigated ,21 but evidence from randomized clinical trials remains limited.22-24 If subjective symptoms are strong, symptomatic treatment involving pain management is necessary. For severe pain, antiarrhythmics, anticonvulsants, antidepressants, etc., have been prescribed, but in many cases, the effect is not adequate. In recent years, pregabalin 25-33 and duloxetine,25-37 which have high analgesic effects on neuropathic pain, have also, became usable for diabetic pain. These were a boon for patients with diabetic neuropathy. Pregabalin binds to calcium ion channels distributed mainly in the nervous system and exerts an analgesic action by suppressing the release of various neurotransmitters. Duloxetine exerts a sedative effect by increasing the levels of serotonin and noradrenaline, which are involved in pain control.
Diagnosis and examination methods
Diagnosis: No symptoms or tests specific to diabetic neuropathy exist. Currently, no diagnostic criteria developed through internationally established consensus are available. Therefore, it is necessary to comprehensively diagnose neuropathy based on neurological symptoms and examination results. The diagnostic criteria (Table 2) of the American Diabetes Association are reasonable and applicable to daily clinical practice.
Diagnosis |
Diagnosis Items |
Purpose |
Possible DSPN |
The presence of symptoms or signs of DSPN may include the following: symptoms–decreased sensation, positive neuropathic sensory symptoms (e.g., “asleep numbness,” prickling or stabbing, burning or aching pain) predominantly in the toes, feet, or legs; or signs-symmetric decrease of distal sensation or unequivocally decreased or absent ankle reflexes. |
Clinical use |
Probable DSPN |
The presence of a combination of symptoms and signs of neuropathy include any two or more of the following: neuropathic symptoms, decreased distal sensation, or unequivocally decreased or absent ankle reflexes. |
Clinical use |
Confirmed DSPN. |
The presence of an abnormality of NC and a symptom or symptoms or a sign or signs of neuropathy confirm DSPN. |
Clinical use research |
Table 2 Definitions of minimal criteria for DSPN.42
DSPN: Distal Symmetric Polyneuropathy
Examination methods: Useful tests for screening diabetic neuropathy include the pain sensation test, vibration sensation test, 10-g monofilament test, and Achilles tendon reflex assessment. Heart rate variability examination is also relatively simple and useful as an autonomic nerve function test. By regularly conducting these tests in daily clinical practice, it is possible to appropriately evaluate the onset and development of neuropathy. These tests are also effective for the early diagnosis of asymptomatic diabetic neuropathy. In addition to relatively easy implementation, these are very useful examinations if the examiners are proficient. However, one problem is that these are qualitative examinations. Meanwhile, for obtaining a confirmed diagnosis, quantitative nerve conduction examination is necessary, but it has low penetration and implementation rates because expensive inspection equipment and a long inspection time are required. We re-emphasize that diabetic neuropathy is a diagnosis of exclusion.
Sensory function test: The extent of sensory neuropathy is evaluated with the pain sensation test (to examine hyperalgesia and attenuation sites using pins), vibration test (to examine the vibration sensation by applying a tuning fork to the ankle), and 10-g monofilament test.38
Tendon reflex: In diabetic neuropathy, tendon reflexes in the lower limbs, especially Achilles tendon reflexes, are often reduced or disappear.
Quantitative tactile examination device: Many patients are unaware of diabetic neuropathy, and the neuropathy testing rate is low because simple or comprehensive examinations have not been developed. Moreover, there are fears that qualitative examinations carried out in daily clinical practice depend on the competence of inspectors in most cases. Meanwhile, the nerve conduction examination, which is a quantitative examination, requires expensive examination equipment and a long inspection time; hence, its penetration and implementation rates are extremely low. We need an examination method that can evaluate DSPN more easily, sensitively, quantitatively, and noninvasively than do conventional examination methods and that can be performed by patients themselves. Therefore, the authors focused on the decrease in tactile sensation of DSPN patients and developed a quantitative tactile sensation measurement device,39 (Figure 1), hereinafter referred to as “the device”).
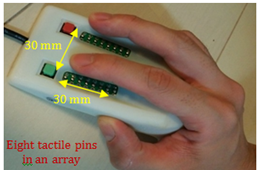
Figure 1 Quantitative tactile sensation measurement device for screening diabetic neuropathy.39
First, we used this device to conduct a pilot study of 15 diabetic patients with a long history of treatment and confirmed a significant decrease in tactile sensation compared with healthy subjects.40 Second, we used this device to conduct a validation test of the DSPN evaluation method in diabetic patients41 According to the criteria for probable DSPN given by the American Diabetes Association; tactile sensation was quantitatively quantified and compared between the two groups. A significant reduction in tactile sensation was confirmed in the DSPN group. Third, we compared tactile sensation between 31 asymptomatic DSPN patients and 32 healthy volunteers. The results confirmed significant tactile reduction in asymptomatic DSPN patients.39 Strength of this device is that it can be easily used even by patients and quantitative results can be reported in a short span of several minutes. Furthermore, this method may be applied not only to diabetic neuropathy but also to peripheral neuropathy in general. Currently, a new device for assessing the lower limbs is also being studied. Applications in diseases other than diabetic neuropathy are also under assessment.42
We briefly present an overview of the current state of diabetic neuropathy. Diabetic neuropathy is not taken into consideration in daily clinical practice because no testing or diagnostic methods have been established. In the future, it is important to establish a simple and quantitative testing method to detect asymptomatic DSPN early and enable aggressive treatment.
This work was partly supported by the Grants-in-Aid for Scientific Research, the Japan Society for the Promotion of Science (No. 24500548 and 17K17925) and by endowments from MSD and Takeda Pharmaceutical Company. The authors would like to thank Enago (www.enago.jp) for the English language review.
Keiji Uchida has a patent of Quantitative tactile examination device. The other authors declare that they have no competing interests.

©2018 Danjo, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.