International Journal of
eISSN: 2577-8269


Opinion Volume 7 Issue 3
Advanced Polymeric Nanostructured Materials Engineering, Graduate School of Engineering, Toyota Technological Institute, 2-12-1 Hisakata, Tempaku, Nagoya 468 8511, Japan
Correspondence: Prof. Masami Okamoto, Advanced Polymeric Nanostructured Materials Engineering, Graduate School of Engineering, Toyota Technological Institute, 2-12-1 Hisakata, Tempaku, Nagoya 468 8511, Japan, Tel +81 52 809186, Fax +815280918641
Received: June 15, 2023 | Published: June 28, 2023
Citation: Okamoto M. Cellular morphology-mediated functional characteristics of breast cancer cells. Int J Fam Commun Med. 2023;7(3):115-116. DOI: 10.15406/ijfcm.2023.07.00322
cancer, poor treatment, radiation and chemotherapy, morphology, tumor progression, biophysical cues
ECM, extracellular matrix; DHM, digital holographic microscopy; Pap, Papanicolaou; ND, maximum nuclear dimension; MHN, maximum nuclear height; AN/AC, nuclear-to-cytoplasmic ratio
Breast cancer is one of the most common malignancies in women and has the highest incidence and mortality of all female malignancies.1 The average 5-year survival rate for breast cancer is reported to be approximately 55% due to the poor treatment outcome of metastatic disease, including resistance to radiation and chemotherapy.2 Given the poor prognosis associated with these cancer treatments, there is a compelling need to address new research into effective cancer treatments.
When a traction force is generated within a cell underlying an extracellular matrix (ECM) containing an artificial matrix, the cell inherently senses the stiffness of the surrounding microenvironment and pulls itself into the matrix in response to the applied force. It exerts a force and the traction force can change the morphology and cytoskeleton of the cell (Figure 1).3,4
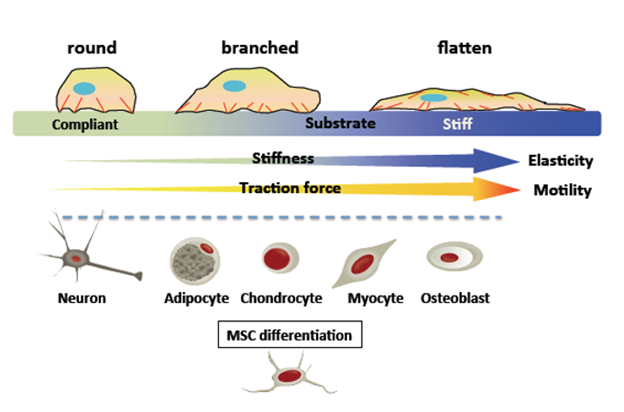
Figure 1 Cells exert a traction force on the underlying substrate and essentially "feeling" this stiffness.2 In general, cells generate greater traction, establish more stable and well-defined focal adhesions, form more defined actin stress fibers (red), and spread more extensively on stiff substrates than on compliant substrates. A soft substrate is beneficial for neurogenic or adipogenic differentiation, a hard substrate is beneficial for osteogenic differentiation, and an intermediate hardness is indicated because "tissue cells feel and respond to the hardness of their substrate". The substrate favors myogenic lineage engagement.3
Microenvironment-mediated tumor progression in artificial matrices explores design criteria for understanding cancer progression mechanisms and metastatic potential. Cancer cells express multiple biophysical cues and epigenetic programs of phenotypic transition during migration. Quantifying and investigating these biophysical cues as morphological changes in cancer cells have applications not only in hematology but also in cancer treatment and diagnosis.5
Recently, the extracted morphological features can also be used to analyze dynamic changes in cells in neurological diseases and cell stress-related phenomena.6 Moreover, multivariate analysis of morphological data suggests that quantitative cytology is a useful adjunct to conventional testing for the selection of new substances.7 Because biophysical cues exist long before cancer develops, they are most useful in determining whether cells will metastasize. With knowledge of potentially cancerous changes in the body, clinicians may be able to initiate preventative measures in the form of lifestyle changes and chemotherapy.
Morphological change in cancer cells and cancer diagnosis
Using digital holographic microscopy (DHM), contrast agent-free morphological analysis and dynamic behavior of cancer cells have been reported.8 A suitable technique for gynecologic cervical samples has been demonstrated as an alternative to cytological Papanicolaou (Pap) testing.9 Pap test normal cell images showed superficial cells with small nuclei. In contrast, abnormal cell images revealed superficial cells with larger, irregular nuclei. The Benzerdjeb group identified DHM criteria that can distinguish abnormal cells. The best diagnostic criteria were maximum nuclear dimension (ND), maximum nuclear height (MHN), and nuclear-to-cytoplasmic ratio (AN/AC). By using ND and AN/AC cutoff values, an automated algorithm can be built to select abnormal cells.
DHM imaging combined with machine learning for data analysis is a powerful tool for distinguishing SW480 colon cancer cells from leukocytes in suspension.10 Furthermore, DHM analysis using deep learning neural networks identifies isogenic cell lines at different metastatic stages11 and distinguishes between healthy and nutritionally deprived cancer cells.12 Furthermore, cancer cell cycle tracking may identify cancer cell behaviors such as morphologically distinct motility patterns. This leads to targeting mechanisms of cancer growth and chemosensitivity.13
Assessment of cell morphology associated with the contractile and stress-resistant cytoskeleton has implications for phasic signals from cell boundaries and/or nuclei. As reported by the Okamoto group in the literature,14,15 cell morphology of different cancer phenotypes provides a quantitative method to distinguish different effects of cancer biomarkers.16
This work was supported by the KAKENHI of the Ministry of education, Sports, science and Technology, Japan.
Author declares that there is no conflicts of interests.

©2023 Okamoto. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.