International Journal of
eISSN: 2381-1803


Review Article Volume 14 Issue 1
1Department of Chemistry, University of the Sciences in Philadelphia, USA
1 Department of Chemistry, University of the Sciences in Philadelphia, USA
2Department of Biological Sciences, University of the Sciences in Philadelphia, USA
2 Department of Biological Sciences, University of the Sciences in Philadelphia, USA
Correspondence: Bela Peethambaran, Department of Biological Sciences, University of the Sciences in Philadelphia, Philadelphia, Pennsylvania, 19104, USA, Tel 2155968923
Received: December 22, 2020 | Published: January 22, 2021
Citation: Leonce C, Patel A, Peethambaran B. The role of oxidative stress and the underlying biological pathways in the pathogenesis of Parkinson’s Disease. Int J Complement Alt Med. 2021;14(1):17-24. DOI: 10.15406/ijcam.2021.14.00528
In this review, we present evidence collected over a decade concerning signaling pathways and pathogenic mechanisms that are associated with oxidative stress in Parkinson’s disease. Parkinson’s is associated with several protein such as α-synuclein and signaling pathways such as Wnt signaling pathway. The review highlights the connection of the Wnt mediated pathway with other biological pathways that are known to have a role in neurodegeneration and the orchestrated role of several proteins in mitochondrial oxidative stress in Parkinsons. We have highlighted neuroprotective agents that eliminate the excess of reactive oxygen species and have a potential to be developed as therapeutics for Parkinson.
Keywords: parkinson’s, oxidative stress, natural treatments for parkinson’s, biological pathways
PD, parkinson’s disease; iNOS, induced nitric oxide synthase, NO, nitric oxide; PUFAs, polyunsaturated fatty acids
Parkinson’s Disease (PD) is a neurodegenerative disease that mainly affects the substantia nigra pars compacta of the brain.1 This is the dopamine and melanin producing section of the brain which controls motor skills and in Parkinsons is shown to manifest with symptoms such as tremors, slow mobility and loss of posture and balance.2 PD is the second most common neurodegenerative disease after Alzheimer’s Disease and affects over one million people worldwide. PD symptoms usually manifest in patients over 50years of age, however, symptoms are seen earlier in patients with genetically caused versions of the disease. Patients with idiopathic forms of PD have unknown cause of disease manifestation but there have been reports of higher cases of PD in persons who work in agricultural and manufacturing industries.3 Generally, increased oxidative stress has been linked to the pathology of PD,4 however, simply treating patients with antioxidants has not been able to fully reverse or alleviate the progression of the disease. Because of this, current PD treatments serve only to slow the progression of the disease and manage symptoms.
Generally, there are two main discussions/models surrounding the pathogenesis of PD, the oxidative stress model, and the genetic mutations model. Though both models focus on different aspects of PD, the consensus is that they both work together to bring about PD pathogenesis.5 This pathogenesis causes damage to the substantia nigra neurons, causes a decrease in dopamine production, increase in Lewy body formation and ultimately cell death.6 A marked difference can be seen in the neurons of patients with PD (Figure 1). With familial PD, its etiology can be pointed towards many gene mutations including; Parkin, DJ-1, PINK1, LRRK2, ɑ-synuclein, to name a few.7 However, there is limited consensus on the etiology of idiopathic PD, and it remains widely unknown. Various mentioned causes for idiopathic PD include brain injury and exposure to various environmental factors such as pesticides and neurotoxins.8,9
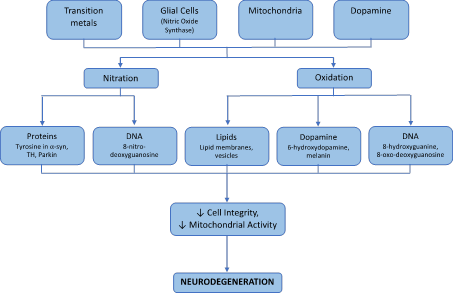
Figure 1 A flowchart that shows how the production of ROS and RNS caused by transition metals, Glial cells, mitochondria and dopamine can affect many different aspects of structure and activity of cells and lead to neurodegeneration.
There are several genes that are implicated in the degeneration of substantia nigra pars compacta. The degeneration is commonly associated with the accumulation of a-synuclein containing Lewy bodies.2 Current studies cannot accurately pinpoint a specific mechanism underlying the neurodegenerative cause in PD; however, several studies show a clear role of oxidative stress, mitochondrial dysfunction, and neuronal inflammation.10 It is clear from current neurobiology research that oxidative stress products damage DNA, lipids, proteins and many vital macromolecules (Figure 1). Lack of remediation and control of oxidative damage is the prime culprit for neurodegeneration. The downstream effects of these macromolecules damage led to decrease in dopamine a level that causes the classical symptoms such as cognitive impairment, depression, mood fluctuations, psychosis, dementia other than the motor complications seen in Parkinson. This review highlights both the oxidative stress and mutation in key proteins that lead to the pathogenesis of PD. Current drugs to treat Parkinson such as Levodopa, cycrimine, carbinoxamine and carbidopa have led to side effects such as tension, apprehension, lack of balance, lethargy which affects patient’s health. Recently, several studies have reported medicinal plant-based therapy. Eight natural compounds that have potential to be developed as therapeutics have been described in detail in this review.
Oxidation/nitration of proteins, DNA, lipids and their building blocks
Uncontrolled production of ROS and RNS causes serious damages to biomolecules. These consequences can be seen in nucleic acids of RNA/DNA, amino acids and specific proteins, various lipids and other key compounds. These alterations in structure based on oxidation and nitration products and formation of adducts have dire effects on metabolism, structure, function and activity of many pathways. ROS and RNS has been known to oxidize or nitrate guanine to 8-hydroxyguanine, 8-nitro-deoxyguanosine and 8-oxo-deoxyguanosine and cause deamination of proteins including the change of cytosine to uracil and guanine to xanthine.11–13 DNA crosslinks between thymine and tyrosine have also been observed.14 These products have been specifically increased in the substantia nigra of the brain and are implicated in mutations that lead to development and progression of PD.
Another key effect of RNS is the nitration of key amino acids, mainly tyrosine to 3-nitrotyrosine.15 Nitration usually occurs directly by nitric oxide or peroxynitrates formed through a Haber Weiss reaction involving transition metals.16 Tyrosine residues are nitrated at the ortho position on the benzene ring and makes it bulkier. The nitrate also adds a negative charge to the protein, changes its pKa from 10.01 to 7.2 and prevents protein rotation because of the bulky group.17 Increased 3-nitrotyrosine can be observed in alpha-synuclein and tyrosine hydroxylase.18,19 Nitration of these proteins have an effect on their activity, their structure and their ability to be degraded by proteasomes. 3-nitrotyrosine also has neurotoxic effects on its own after conversion to 3-nitro-4-phenylacetate by amino acid decarboxylase. Through this mechanism, 3-nitrotyrosine causes a decrease in intracellular dopamine levels.20 The compound effect of 3-nitrotyrosine and the nitration of key proteins and enzymes in the SNc contributes to the degeneration present in PD patients.
ROS and RNS also have other effects including decrease in polyunsaturated fatty acids (PUFAs) and a concurrent increase in oxidation products such as malondialdehyde, 4-hydroxy-2-nonenal and similar byproducts.21,22 An increase in protein carbonyls have also been observed.23,24 Adducts of cysteine and GSH to levodopa, dopamine and 3,4-dihydroxyphenylacetic acid have been observed.25 The formation of quinones and catechol-quinones have also modified cysteine and cysteinyl compounds.26 Also, a general decrease in GSH and a subsequent increase in ROS and inhibition of mitochondria has been observed.27,28 These effects have been specifically observed and measurable in the SNc of PD patients and can be markers for the development and progress of the disease.
Sources of ROS/RNS
Mitochondria have been known for a long time to be a source of ROS in living organisms through the process of oxidative phosphorylation. Production of ROS in mitochondria is not innately negative and has been involved in maintaining cellular homeostasis and as signals for many pathways.29 Mitochondrial ROS production is based on a balance of ∆p, NADH/NAD+, CoQH2/CoQ and oxygen concentration.30 An imbalance in any of these systems can trigger overproduction of ROS leading to oxidative damage and cell death. Mitochondrial Ca2+ also plays a role in the stimulation of oxidative phosphorylation with an increase in Ca2+ causing an increase in respiration.
The major ROS produced in mitochondria is superoxide which then dismutates to produce hydrogen peroxide. These ROS can be damaging on their own but can also go on to produce hydroxyl radicals and react with nitric oxide to produce peroxynitrite.16
NADH dehydrogenase (Complex I) and Complex III are the major sources of superoxide in the mitochondria and inhibitors such as rotenone, antimycin and cyanide increase superoxide production in these complexes.31 Complex I releases superoxide on the matrix side of the mitochondria and Complex III on the cytoplasmic side.32 Under physiological condition, manganese-SOD can sequester and neutralize the superoxide produced. If unchecked, ROS can inhibit the electron transport chain, and release iron from the iron-sulfur clusters and other iron storage units in the mitochondria.33 The free iron can take part in Fenton reactions and produce more ROS. It is also interesting to note that the substantia nigra has a high level of iron storage and is more susceptible to imbalances in mitochondrial ROS.34 Coupled with the high rate of metabolism in neuronal cells, maintaining ROS levels in the mitochondria continues to be a key target for treatment for PD.
Glial cells
Glial cells are the most abundant cells in the brain and serve many roles. They have been implicated in PD because of their noted activation in PD patients and their ability to produce ROS and RNS and neuronal inflammation.35 Microglial activation has been shown to cause an increase in anti-inflammatory cytokines including IL-6, TNF-𝛼 and INF-𝛾.35-37 Glial cells have been shown to be activated by extracellular a-synuclein, neuromelanin and external toxins. A-synuclein is phagocytosed by glial cells where it then activates NADPH oxidase to produce ROS.38 Neuromelanin acts as a chemoattractant, drawing microglial to cells which have been damaged and released their stored neuromelanin. Initial neuronal apoptosis also releases activated MMP-3 which activates microglia and may cause damage to remaining healthy cells in the direct vicinity.39-41 Glial cells can also be activated by rotenone, paraquat, liposaccharides and diesel exhaust.42 ROS and RNS produced by glial cells occur through a cascade that starts with the production of induced nitric oxide synthase (iNOS) which then produces nitric oxide (NO). The NO reacts with cellular superoxide to form peroxynitrite. This peroxynitrite then goes on to nitrate amino acid residues in key proteins such as 𝛼-synuclein, parkin and tyrosine hydroxylase. The nitrosylation of these proteins causes a decrease in their activity and an increase in PD development.17,26,43,44
Dopamine oxidation
The production of dopamine is key for the function and maintenance of fine motor skills. Dopamine is formed in SNc by the hydroxylation and decarboxylation of tyrosine. Normally dopamine is sequestered in synaptic vesicles for use and is protected from metabolism. However, free dopamine is subject to metabolism by monoamine oxidase B, catechol-o-methyltransferase and aldehyde dehydrogenase to homovanillic acid.45 Dopamine can also undergo autoxidation to make byproducts that are toxic to cells. It is these oxidation products that contribute to the selective deterioration of SNc neurons and the development of PD. Dopamine autoxidation causes the production of aminochrome/dopachrome through the production of DOPA-semiquinone and DOPA-quinone steps.46 These quinones can be formed without the need of catalysts and enzymes but are affected by pH.47 Each step has the ability to produce ROS in the form of superoxide and hydrogen peroxides. Dopamine oxidation can also be catalyzed by peroxidase enzymes and metals such as copper and iron to produce dopachrome products.48-51 One key oxidation product of dopamine is 6-hydroxydopamine, which is shown in high levels in PD patients and is used as a model for PD in research.52,53
The overproduction of dopamine oxidation products has numerous effects on the neuronal cell. Dopachrome causes an increase on the production of melanin which was shown to cause an increase in lysosomal dysfunction and decreased lysosomal proteolysis and a concurrent increase in alpha-synuclein aggregation.54 Oxidative products also formed adducts directly with alpha-synuclein and increased likelihood of aggregation.54,55 Dopamine oxidation also causes a decrease in mitochondrial potential and activity and mitochondrial phosphorylation.56,57 A decrease in mitochondrial activity causes an increase in ROS and apoptotic signals. Therefore, management of dopamine oxidation and its products could be a viable target for treatment for PD.
Tyrosine hydroxylase
Peroxynitrite and nitrogen dioxide are able to nitrate the tyrosine residues on tyrosine hydroxylase (TH) and inhibit enzymatic activity.19,23 Since TH is the limiting step in dopamine production, inhibition of the enzyme means a decrease in dopamine and a subsequent increase in PD development/symptoms. Nitration is likely to occur on one of the 15 residues in the active site of TH and the change in size, pKa and charge of the residues would have a great effect on enzyme activity by limiting the ability of the enzyme to be phosphorylated.26 Peroxynitrite can also react with the copper in SOD to produce toxic nitronium dioxide ions which can then react with the proton on SOD’s only tyrosine to form 3-nitrotyrosine.58
Alpha-synuclein
A-synuclein can be nitrated at tyrosine residues to form nitrosylated a-synuclein and is a key target of increased nitric oxide moieties in neuronal cells.43 Nitrosylated a- synuclein shows decreased solubility, increased fibrillization and slower degradation of the protein when exposed to 20 S and Calpain I proteasomes.17,43 A decrease in 𝛼-synuclein monomers to bind to vesicles was also observed.17 These effects play a key role in PD pathogenesis as an increase in a-synuclein causes aggregation which is a key signal of PD progression. A decrease in 𝛼-synuclein binding to lipid vesicles causes a decrease in the ability for neurotransmitters, such as dopamine, to be released from neuronal cells and illicit their appropriate reactions for neuronal excitation and maintenance of motor functions.17
Parkin
Parkin is another protein that is affected by nitration. Parkin is an E3 ligase responsible for adding ubiquitin to substrates for degradation. Parkin undergoes s-nitrosylation by nitric oxide. This diminishes the ligase activity of parkin and its protective effect by allowing an increase in 𝛼-synuclein aggregation.44,59
PI3K/AKT signaling pathway: Recent studies have also drawn attention to the possible role of the serine threonine kinase, AKT having a mechanistic role of signaling in defective neuronal cells (Figure 2). There are several key activators of the AKT pathway via phosphatidylinositol 3-kinase (PI3-K) caused by increases or decreases to growth factors, cell stress, and insulin60 this causes an upregulation of phosphatidylinositol (3,4,5) P3 (PIP-3), which then leads to recruitment of Akt to the plasma membrane, where phosphorylation at serine-473 and threonine-308 induces Akt activation. Upon activation Akt has many binding sites which all play key roles in the functionality of neuronal cells such as metabolism and cell survival, these substrates include the protein complex mTOR, CREB, BAD, and GSK3b. The impact AKT phosphorylation has on the downstream proteins is incredibly important. Many drug targets that could potentially alleviate PD symptoms have been shown to be neuroprotective via AKT activation.61 Expectedly, expression of active phosphorylated AKT protects against induced dopaminergic cell death brought on by 6-hydroxydopamine (6-OHDA).62 Previous studies have also elucidated that decreased AKT activation brought on by the induction of the RTP801 protein, increased PD pathogenesis.63
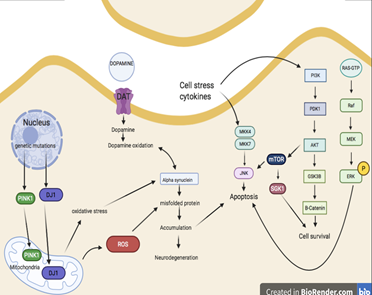
Figure 2 Summarizes the biological pathways that play a key role in alleviation of oxidative stress in Parkinson’s.
Mitogen-activated protein kinase signaling pathway: The mitogen-activated protein kinase family (MAPK) is an old, evolutionarily highly conserved family of serine/threonine protein kinases liable for intracellular signaling. The MAPK proteins64 survival, cell death, stress response and gene expression.65 The extracellular signal-regulated kinases 1 and 2 (ERK1/2) signaling pathway is a fundamental moderator of various cellular processes, this pathway is activated by several different growth factors, G protein-coupled receptors (GPCRs), insulin, and stress factors.65
ERK 1/2 signaling cascade is heavily involved in neuronal death, which is a major cause in all diseases of the neurodegenerative nature including PD.66 In regard to PD there are several ERK 1/2 processes that affect PD pathology such as mitochondrial dysfunction, inflammation, neuroprotection, oxidative stress, cell survival and apoptosis. In terms of mitochondrial dysfunction, recent studies have found that PD patients have a mild deficiency in the mitochondrial complex I, within the substantia nigra.64 p-ERK 1/2 was also found in the mitochondria of actively degenerated neurons originating from patients with Lewy body dementia and patients with PD.67 There is an orchestrated signaling of all these proteins that affects the oxidative stress (Fig2).
Majority of PD cases are idiopathic however a small percentage of them result from highly specific genetic mutations. Familiar or genetic PD typically presents with the same clinical symptoms as idiopathic PD with the key difference being earlier onset of the disease.68 There are several genes that code for SNCA (SNCA), ubiquitin carboxyl terminal hydrolase L1 (UCH-LI), leucine rich repeat kinase 2 (LRRK2) are heavily involved in the autosomal dominant forms, where genes coding for parkin (PRKN), ATPase type 13A2 (ATP13A2), PTEN-induced putative kinase 1 (PINK1), and PD associated protein DJ-1 (PRKN7) are heavily involved in autosomal recessive forms of PD.69 Many of these genes play significant roles in mitochondrial function, protein ubiquitination, and oxidative stress, these genes also interact with each other. SNCA was the first PD gene to be mapped. This led to the discovery that the encoded protein is the primary component of Lewy bodies. SNCA is a relatively small protein with a kDa of 14.46, when overexpressed via triplication and duplication of the gene, SNCA, is associated with DA neuronal toxicity in humans with PD.69
Mutations in the gene encoding for LRRK2 have been recently linked with late the late-onset, autosomal dominant form of familial PD. The protein LRRK2 is often looked into as a promising target for therapeutic treatment of PD. Much like SNCA, the precise function of LRRK2 remains largely unknown. LRRK2 is complex and unusually large, with many protein-interaction, and enzymatic domains.70 These multiple domains can potentially be targeted by mutations in familial PD. In recent studies LRRK2 increased kinase activity is often linked with neuronal cell death, cellular toxicity and gain of function point mutations, which is observed in both idiopathic and familial PD. Although LRRK2 mutations do not aide in the formation of Lewy bodies, they do however constitute a large share of genetic mutations related to familial PD.71
PINK1, DJ-1 and PRKN are responsible for the autosomal recessive form of PD. PRKN is a cytoplasmic protein primarily, that functions in tagging proteins for proteasomal degradation. Mutations in PRKN are thought to weaken its ability to interact with E3 ubiquinating enzymes, this impaired interaction leads to a build-up of proteins that are unable to be cleared leading to neuronal cell death overtime.72 PRKN, PINK1 and DJ-1 are all involved in pathways that regulate mitochondrial function, clearing, morphology and damage in PD.73 PINK1 is a mitochondrial protein that regularly interacts with DJ-1 to modulate the activity of PRKN to aide in protein ubiquitination. When a loss of function mutation occurs in PINK1 it hinders the role of the protein in a mitochondrial quality control pathway, leading to eventual mitochondrial dysfunction and neuronal cell death.74 DJ-1 functions to maintain the functionality and integrity of the mitochondria against oxidative stress.75 There are various other genes implicated in their respective role in the etiology of PD, however, there is relatively little known regarding their actual function. Although there has been considerable progress in the identification of genes implicated in PD, the functionality of these genes needs to be further investigated.
The discovery of levodopa has allowed for great strides towards the treatment of PD and extension of patients’ life. However, because of the side effects of continued use and its inability to cure PD, natural products have been used, individually and in conjunction with drugs, to treat PD and lessen its effects. Natural products have been known to decrease tremors and increase mental function. Plants such as Mucuna pruriens, Hyoscyamus niger, Sida cordifolia and Withania somnifera have been used extensively to treat PD. M. pruriens seed extract showed effect as a metal chelator and antioxidant. H. niger seed extract had an anticholinergic effect and the active compounds isolated were hyoscine and hyoscyamine. S. cordifolia and W. somnifera root extracts had antioxidant effects. Other plants showing promising effects as antioxidants and mitochondrial protectants include C. asiatica, B. monnieri, S. suaveolens, T. cordifolia and N. jatamausi. Other plants consumed, that focus on consuming high amounts of antioxidants, such as Gingko biloba, green teas and berries, have also been used to help treat and slow the progression of PD (Table 1).76,77
|
Compound name |
Target |
Mechanism |
References |
|
N-acetyl cysteine |
Provides cysteine to maintain GSH levels Antioxidant |
Acts mainly as a pro-drug to provide cysteine for the production of GSH. Maintains high GSH levels and protects against OH-, H2O2 and HOCl. |
78– 83 |
|
EGCG |
Antioxidant |
Protects TH producing cells and TH activity which allows for production of dopamine and metabolites. Decreased levels of nNOS and iNOS. Decreased fibrillogenesis of 𝜶-synuclein. |
84–89 |
|
CoQ10 |
Increased mitochondrial function Antioxidant |
Protects mitochondria by maintaining integrity and decreasing ROS formation. |
90–95
|
|
Withanone, Withafarin, Withanolides (Withania somnifera) |
Antioxidant |
Increase SOD, CAT and GPx activity Free radical scavenging Increase catecholamine levels Maintains mitochondrial integrity |
96–102
|
|
Ginsenosides (Panax ginseng) |
Antioxidant Anti-inflammatory |
Reduced inflammation by inhibiting Nitric oxide synthase and decreasing levels of pro-inflammatory cytokines. Increased cell viability |
103–107
|
|
Ginkgolides (Ginkgo biloba) |
Increased dopamine levels Antioxidant Increased mitochondrial function |
Increased dopamine levels through regulation of dopamine genes and transcription factors Maintains mitochondrial potential and limits mitochondria-related ROS production |
108–111
|
|
Baicalein, Baicalin (Scutellaria baicalensis) |
Antioxidant Increased mitochondrial function Reduces levels of Apoptosis Increased dopamine levels |
Protects mitochondrial membrane from insult Reduces levels of ROS through activation of ROS-scavenging enzymes Reduces apoptosis through management of caspase and JNK |
112–115 |
|
Hyoscyamus niger
|
Antioxidant |
Increase ROS-scavenging enzyme activity, reduced ROS |
116–118
|
Table 1 Role of natural neuroprotective compounds that have potential to treat Parkinson’s Disease
The mechanisms involved in pathogenesis and progression of PD is not fully understood but there is overwhelming evidence that maintenance of redox potential is important for neuronal survival. Any disruption in the mitochondrial potential disrupts the cellular homeostasis, which in turn causes more ROS production leading to neuroinflammation and degeneration. The review attempts to consolidate key signaling pathways, and proteins that can be targeted to develop therapeutics for Parkinson’s. The conventional medicine is effective but has several side effects; hence developing small molecules that can modify these molecular targets selectively can be explored.
This study was supported by funds from the Endowed Funds for the Roth Chair in Pharmacognosy awarded to Dr. Bela Peethambaran by The University of the Sciences.
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
None.

©2021 Leonce, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.