International Journal of
eISSN: 2381-1803


Review Article Volume 7 Issue 6
1Department of Cardiology, Institute of Hospitalization and Care with Scientific Address, Italy
1Department of Cardiology, Institute of Hospitalization and Care with Scientific Address, Italy
2CRESO, School of Osteopathic Centre for Research and Studies, Italy
3Foundation Polyclinic University A, Gemelli University Cattolica del Sacro Cuore, Italy
4Sapienza University of Rome, Italy
Correspondence: Bordoni Bruno, Foundation Don Carlo Gnocchi IRCCS, Department of Cardiology, Institute of Hospitalization and Care with Scientific Address, S Maria Nascente, Via Capecelatro 66, Milan 20100, Italy, Tel 0039.02.3496300617
Received: July 05, 2017 | Published: July 11, 2017
Citation: Bordoni B, Marelli F, Morabito B, Sacconi B (2017) Proposal for a New Manual Evaluation Scale for the Diaphragm Muscle: Manual Evaluation of the Diaphragm Scale – MED Scale. Int J Complement Alt Med 7(6): 00242. DOI: 10.15406/ijcam.2017.07.00242
The main functions of the diaphragm are altered in the presence of respiratory diseases, due to a reduction in muscle mass and electrical coordination, and a non-physiological adaptation of the intrinsic protein structure. Patients with COPD or OSAS, and people weaned from ventilation machines follow a rehabilitation path and physiotherapy to recover the diaphragm strength, combining manual and osteopathic therapy with specificic techniques which can be directly used on the rib muscle. The operator manually evaluates the mobility of the diaphragm before and after physiotherapy; the initial evaluation is useful to address the training, and the final one to evaluate the outcome.
In literature a manual assessment of the less mobile areas of the diaphragm is currently missing, these information would be vital for the operators, in order to decide which muscle areas need to be treated. The scale proposed in the present article can be useful to address the treatment on specific affected areas and can improve informational exchange on the patient's specific needs between different professional persons. The article is the first step to present the scale as a hypothesis. Subsequently, we will make a second article to validate the scale with objective clinical tools.
Keywords: diaphragm, COPD, OSAS, osteopathic, physiotherapy, chiropractic
Chronic obstructive pulmonary disease (COPD) is major public health problem, causing significant mortality and morbidity worldwide.1 The WHO estimates a current mortality rate of around 64 million people; it has been estimated that, in 2030, COPD will represent the third cause of mortality in the world.1 It is a progressive disease characterized by chronic inflammation and dysfunction of the upper airways.2
The inspiratory dysfunction of the diaphragm is a key element in patients with COPD. The progressive limitation of the airflow in COPD patients causes a pathological adaptation of the diaphragm, although the reasons for these changes are not fully clear. These changes in position adversely affect the exercise tolerance; more in detail, the dome of the diaphragm is lowered, in inspiratory position.3 The contractile force is decreased, with electrical and metabolic alterations. The muscle thickness is increased, especially on the left side, with decreased mechanical excursion, probably due to fibers’ shortening.4,5 A decrease of anaerobic type fibers (type II) and an increase in aerobic fibers (type I) is observed; this process progressively increases with the pathology worsening.5 The increase in the oxidative process, however, does not correspond to an improvement of diaphragmatic function. The rate of detectable myosin decreases, resulting in altered sarcomeric organization and further decreasing of the contractile strength.5 The phrenic activity is abnormal, presumably due to the nerve stretching caused by the chronic lowering of the diaphragm, resulting in such a neuropathy.6 The exercise intolerance in patients with COPD does not correlate with the common functional indexes (forced expiratory volume in 1 second - FEV1); rather it is the peripheral muscle adaptation, including that of the diaphragm, to have a heavy influence on the symptomatic scenario.7
Obstructive sleep apnea syndrome (OSAS) is the most common form of respiratory disorder found in the stage of sleep, involving 2-4% of the population worldwide.8 This disorder is characterized by repetitive collapses of the upper respiratory airways and recurrent apnea, leading to different systemic side effects, including cardiovascular, metabolic and cognitive diseases.8 The chronic intermittent hypoxia found in OSAS leads to negative peripheral adaptations of the respiratory muscles, as well as in those patients hospitalized for pneumonia, pulmonary edema, pulmonary fibrosis and other diseases where intermittent hypoxia is detectable; adaptations occur for short periods of time (4 days), and if the disease persists, such effects will worsen.9 Animal models on mice subjected to intermittent hypoxia for 4 days were evaluated, and autophagy processes and atrophy were detected in the muscle, with a phenotypic change to the most aerobic contractile fibers (MHCI), and an initial loss of contractile strength.9 In another study in which hypoxia was induced intermittently and for 7 consecutive days, the diaphragm muscle showed a metabolic and phenotypic change of the muscle fibers; in this model, there was a change of MHCI-type fibers to the MHCII ones, so from an aerobic condition toward a more anaerobic state. Human model studies are discordant too, with some authors reporting that the diaphragm has a reduced contractility in patients with OSAS, while others do not agree.10–13 We know that the electrical coordination between the diaphragm and the tongue is lost, especially in case of respiratory efforts.14 One of the reasons is can be related to the decreased blood flow to the brain areas managing the breathing and coordination among the various motors districts (red nuclei districts, corticospinal motor fibers, pons midline, medial lemnischi), demonstrable in patients with central OSAS.15 These alterations negatively affect the lowest communication paths, such as the motor nuclei of the XII nerve in the bulb and the pool of phrenic medullary neurons; the muscles of the upper airway lose tone, and the diaphragm will not contract in the most adeguate timing.15 We have not data on the preferred position of the diaphragm in patients with OSAS.
Mechanical ventilation (MV) is one of life-saving strategies for critical health patients in intensive care unit; in these patients the respiratory system is able to work well due to different pathological causes and conditions (stroke, heart failure, post-operative infections, etc…).16 One of the first side effects (occurring after 5-6 days) is the weakness of the diaphragm muscle, with a loss of contractile force of about 32%.17 As a consequence, morbidity and mortality increase as well as the difficulty in weaning from the MV and the number of new admissions in intensive care.17 Causes are not completely clear. A phrenic neuropathy can occur, pointing out the fact that it is not a central issue leading to the strength reduction.17 Muscle mass is reduced by approximately 25% after a week, with atrophy, altered organization of sarcomeres, autophagic phenomena, and increased catabolism of diaphragmatic muscle fibers.16–18 Other alterations occur, such as a decrease in the mitochondrial function and in the ability to manage (capture and release) calcium in the sarcoplasmic reticulum, further affecting diaphragmatic contractility.18–20
Respiratory rehabilitation improves bilateral diaphragmatic motion (using fluoroscopic imaging) in COPD patients and the performance, the structural and metabolic characteristics of the respiratory muscle (muscle strength (PI(max),.cm H2O); endurance (Inspiratory Threshold Loading,.kPa), exercise capacity (Borg Scale for Respiratory Effort,. modified Borg scale), Work Rate maximum (watts), and dyspnea (Transition Dyspnea Index).21–23
Several studies valuated the effects of manual therapy and Osteopathy in COPD. The approach varies widely, both in terms of techniques and time management: thoracic spinal mobilization, lymphatic drainage or pump, diaphragmatic release trigger points, massage, articulation techniques for the ribs, myofascial release to the thoracic outlet, sub-occipital decompression, and muscular stretching.24–27 While the results of the various respiratory parameters have been generally positive, we do not have enough information to draw definitive conclusions.
A study showed that the combination of rehabilitation and osteopathy leads to better results than any single approach (increase in FEV1, 6MWT, and decrease in RV).28
A recent study on COPD patients showed that parameters such as mobility, exercise capacity, maximal respiratory pressure, and vital capacity were improved by working the anterior chondro-costal arch with the manual diaphragm release technique.29
Rehabilitation to strengthen the upper airways remains controversial.9 Many patients are submitted to continuous positive pressure ventilation (CPAP), while for patients suffering from hypoventilation (eg obesity or neuromuscular pathologies) BPAP is generally used.30 Not all the patients are able to benefit from this instrumental system because it is difficult to be tolerated all night long, reducing the desired results, even though it is considered a gold standard treatment.30,31 Another approach commonly associated with CPAP and BPAP is physical activity, particularly light to moderate endurance. It improves the apnea-hypoapnea index (AHI), VO2max and TTE80% Wmax (time through exaustion at 80% cycloergometer), the Oxygen Desaturation Index (ODI) and Body Mass Index (BMI), as well as the quality of sleep.32
Myofunctional therapy is a set of exercises aimed at the functional restoration of the tongue and all the relevant structures, such as the muscles of the mouth, supra- and infrahyoid areas, the pharyngeal area.33 These are noninvasive exercises that can be associated with CPAP and physical training, which can improve contractility, proprioception and strength; they improve various parameters recorded in the polysomnography (arousal index, minimum Sa02, time duration Sa02 <90%, sleep efficency, total sleep time).31 They also improve other indexes, such as AHI and ODI, and the snoring intensity.31 Another rehabilitation method to implement the force of the muscles of the oropharyngeal area is the neuromuscular transcutaneous stimulation, in combination with myofunctional therapy exercises. They improve the indexes of polysomnography, as well as the AHI.34
With regards to the manual treatment in OSAS patients, particularly osteopathic manipulative treatment (OMT), only one study performed on children with OSAS demonstrated improvements with polysomnography, such as a decrease in the number of night time apnea.35 However, the manual techniques performed have not been clearly described and the patients number was small (15 in the treatment group).35
Physiotherapy for MV weaning is based on an endurance and anaerobic inspiration training (strenght training) training designed to improve contractile function, ventilation time without assistance, survival, and reduce re-hospitalization. Patients must be collaborative. A gold standard protocol is not described yet in literature; the performed trials show some improvements in the inspiratory strength against resistance (from a minimum of 15/25 cmH2O to a maximum of 54 cmH2O), and in the contractile duration (improvement of the fatigue resistance index (FRI)), but with different rehabilitation pathways.17,36,37 More extensive trials are needed to confirm the absolute safety of the inspiratory rehabilitation in this type of patients and to determine the best physiotherapic strategy to be employed.36,37
Osteopathic Manual Treatment (OMT) for these patients is very variable (myofascial release, balanced ligamentous tension, muscle energy, soft tissue, and inhibition).38 We do not have statistical data regarding hospitalization time and diaphragmatic performance with OMT, thus it is not possible to draw conclusions on whether or not to recommend this approach.
The normal position of the diaphragm can be seen on chest radiography. On the anterior-posterior projection, the dome of the right hemi-diaphragm is located at the level of the 5th-6th rib for what is considered the anterior part, while the posterior one usually lies at the level of the 10th rib.39 The left hemi-diaphragm is slightly higher (about one intercostal space).40 In 10% of people, both domes have the same height, thereby making it difficult to differentiate in the lateral projection.39 Computed tomography and magnetic resonance imaging are also useful in the morphological evaluation of the diaphragm, even though they are used only as secondary analyses, owing to higher costs and availability restrictions.39 Fluoroscopy and ultrasound imaging are techniques used for the real-time evaluation of the moving diaphragm, despite being affected by visibility limitations.39 The ribs open out laterally in the caudal direction during inspiration and the opposite occurs during expiration.40 With aging, the diaphragm becomes thinner and more frequently located in the expiratory position, especially in males.40
In literature there is not a manual diaphragm evaluation scale. It is not possible to share the information handled by the therapist during palpation of the diaphragm muscle with other colleagues in order to create a multidisciplinary rehabilitation strategy. To achieve this kind of therapeutic process, there is a need for an assessment scale highlighting the most dysfunctional areas of the diaphragm. In this way, a more accurate pre- and post-treatment evaluation may be performed by the therapist and the treatment may be more focused on the respiratory needs of the patient.
The human touch can distinguish any slight variation, measurable in microns.41 We strongly believe that it is possible to train therapists to perform an appropriate palpation, in order to check the mobility and function of the inspiratory muscle and to obtain additional clinical information on the therapeutic approach before and after physiotherapy.42
It is important to remember that, as with other therapeutic approach, whether manual or otherwise, conclusive scientific evidence is not available for every existing treatment; however, this does not mean that the therapy is invalid, as otherwise there would be no new developments or improvement in rehabilitative treatments starategies.40 In this regard, we wish to reiterate that evidence-based medicine (EBM), which originated in the second half of the 19th century, is based on individual clinical expertise, best external evidence, patient values, and expectations:
“External clinical evidence can inform, but can never replace, individual clinical expertise, and it is this expertise that decides whether the external evidence applies to the individual patient at all and, if so, how it should be integrated into a clinical decision.43
The MED Scale is presented in a single sheet and as a working hypothesis. On the top and in the middle of this sheet there is the name of the scale; to the left there is an image of the diaphragm, with numbers representing the areas of palpation; to the right the numeric values to be referred to when determining the mobility or restriction of the different diaphragm structures. In the lower part of the sheet, on the left, the recommended palpatory succession is showed, numerically organized, with the numbers corresponding to those on the image of the diaphragm previously described. Immediately to the right, there are spaces (4) to record if the dysfunction is right or left, and empty spaces to score the value found during the palpatory iter. The table where the right or left area is represented respects the specularity of the patient, as the operator is standing, and evaluates with his right hand the left area and vice versa. In this way representing the area with dysfunctions is faster and less confusing. Exceptions are the xifoid area as it is considered a single portion, and medial ligaments, because they intersect on the back-lumbar vertebral bodies (Figure 1).
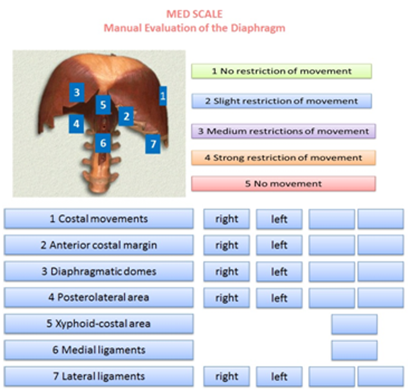
Figure 1 The MED Scale is presented in a single sheet. The MED Scale is used to highlight the precise areas where diaphragm muscle has a limitation on its excursion during an unforced respiratory action.
The MED Scale is used to highlight the precise areas where diaphragm muscle has a limitation on its excursion during an unforced respiratory action. The greater amplitude of motion corresponds to its contractile force and muscular thickness, thus a decrease is equivalent to a loss of contractility and volume.44,45 The scale uses 5 measurements, 1 to 5, form the absence of movement limitations (1) to the complete absence of motility (5), perceived by palpation. Value 2 indicates that motion is present, but there is also a diminished but slight motion excursion, both to passive examination and during operator’s solicitations, as described below. At this stage, if the patient forces the breath at the operator's request, such limitation disappears. Value 3 means an average motion limitation of the diaphragm, with reduced excursion, and tissues are perceived as rigid at palpation. This tissue condition is often synonymous of lack of elasticity and possible fibrosis.42 If a patient is asked for a forced inspiration, the previously assessed palpatory condition does not change. Value 4 points out that the diaphragm has more important movement restrictions. In this condition, the restriction correlates with the severity of the disease, as pulmonary volumes determine the position of the diaphragm and its function.46 Even with this assigned value (4), forced breathing will not alter the assessed restriction. The manual evaluation is performer on the right portion and then repeated on the left one.
In this evaluation, the patient is in a comfortable supine position. In the first step, the costal movement is assessed; this should consist of lateralization during inspiration, with a caudal direction, and the opposite during expiration.47 In the case of dysfunction of the diaphragm, this costal movement is usually limited.40 The hands must be gently placed on the lateral sides of the costal margins to receive palpation feedback of the costal behavior during breathing (Figure 2).
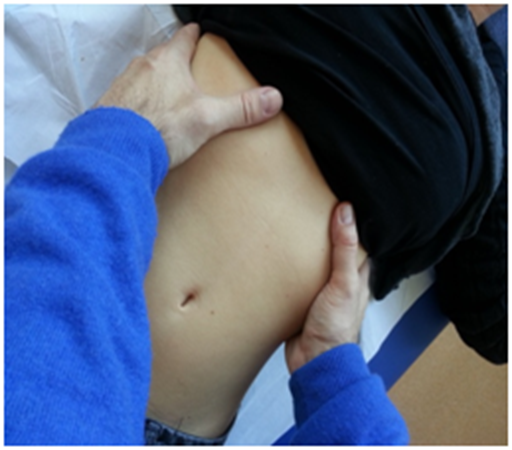
Figure 2 The hands must be gently placed on the lateral sides of the costal margins to receive palpation feedback of the costal behavior during breathing.
In the following evaluation, the hands can be held anteriorly on the costal margins, with the thumbs being at the level of the margins and the other fingers placed across the upper ribs. As the diaphragm falls and rises during inspiration and expiration, respectively, this manual position can be used to assess the diaphragmatic excursion (Figure 3).
To evaluate the diaphragmatic domes, the operator’s forearm has to be held parallel to the abdomen of the patient, with the thenar and hypothenar eminences of the hand at the level of the anterior margin of the costal arch; a gentle push must be performed in the cranial direction, so as to record the elastic response of the tissue, for both right and let sides (Figure 4). As usually observed in manual tests, the elasticity of the tissue is reduced when a reduced movement is obtained in response to the applied stimulus.42
To evaluate the posterolateral area, the hand must be held as previously described for the domes, but with the forearm forming a 45° angle with the patient's abdomen; a gentle push must be applied obliquely, following the line of the same forearm (Figure 5). This portion of the diaphragm shows higher movement excursion during respiration and has a more vertical inclination than the domes.40
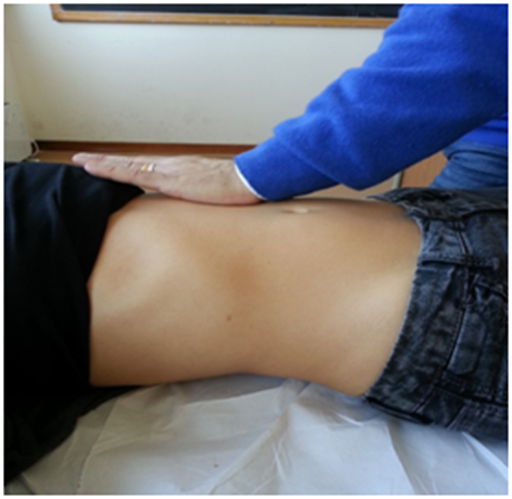
Figure 5 To evaluate the posterolateral area, the hand must be held as previously described for the domes, but with the forearm forming a 45° angle with the patient's abdomen; a gentle push must be applied obliquely, following the line of the same forearm.
The evaluation of the xyphoid-costal area is used to assess whether there is regular elasticity of the tissue during inspiration and expiration, which is necessary for normal breathing; in fact, this area is usually more rigid in cases of abnormal diaphragmatic activity.40 The hand and the forearm are positioned as in the evaluation of the domes, but in the xyphoid area; a gentle push must be applied cranially (Figure 6).
For medial ligaments, the spinal elasticity needs to be evaluated, with the patient being supine. The operator should hold the last phalanges of the fingers (of one or both hands) placed in the interspinous spaces of D11 and D12; by using a gentle push towards the ceiling, a passive extension of the vertebra is obtained, in order to deduce information on their elasticity (Figure 7). The same technique is used to evaluate the lumbar vertebral bodies up to L4.40 The medial ligaments play an important role in the mechanics of the dorso-lumbar region, in synergy with the abdominal wall muscles and the thoraco-lumbar fascia.40
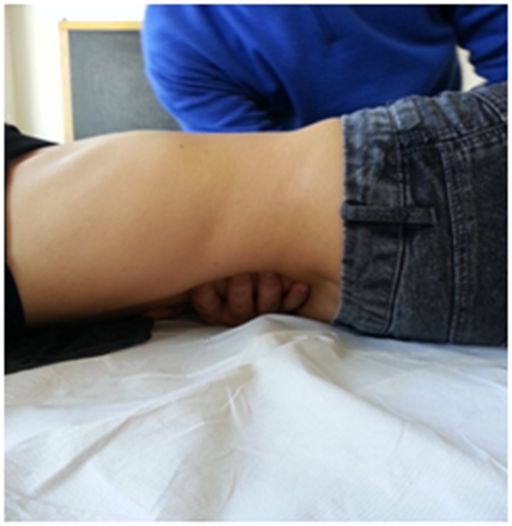
Figure 7 For medial ligaments, the spinal elasticity needs to be evaluated, with the patient being supine.
The lateral ligaments are evaluated by actively involving the last rib. On the opposite side of the rib to be evaluated, the body of the rib must be held with one hand, and a gentle traction must be performed toward the operator (Figure 8). The lateral ligaments play an important role in managing the tension affecting the diaphragm and the thoraco-lumbar fascia.40
MED Scale is a new proposal for manual evaluation for diaphragm muscle, the only one currently existing in the scientific literature. The main purpose of this manual assessment is to understand whether there is a restriction of movement in a specific area of the diaphragm, and to effectively plan a manual treatment focused on the dysfunctional area by combining the manual approach to the conventional rehabilitation process. Having the possibility of obtaining a common exchange of information through this scale should speed up decision-making among different professional figures.
In the next few years, we will try to validate the MED Scale by further research with instrumental means (eg ultrasound), or with the support of radiological evidence, to find out whether the changes experienced by the operator with palpation have an impact with the objective analysis of medical devices. Validating a diaphragm evaluation scale will be a great help in daily therapeutic practice.
In literature there is not yet a manual diaphragm evaluation scale. The present article proposes and describes a hypothesis of valuation scale, in line with the evaluation approach proposed in our previous article.40 MED Scale may permit to collect vital information to support the various operators in the targeted choice of diaphragmatic muscle area to be treated. This scale, proposed as a work hypothesis, unique in its kind, will serve as a basis for comparison between different professional figures, improving the exchange of information on the patient's specific needs, permitting a more focused manual treatment on the diaphragm.
None.
The author declares that there are no conflicts of interest.

©2017 Bordoni, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.