International Journal of
eISSN: 2381-1803


Research Article Volume 11 Issue 5
1School of Biomedical Sciences Charles Sturt University Boorooma St, Australia
2Department of Pharmacy, University of Science and Technology Chittagong-4202, Bangladesh
3Gynae and Obstetrics Department, Chittagong Medical College Hospital, Bangladesh
4Bangabandhu Sheikh Mujibur Raham Science and Technology University, Bangladesh
5Department of Environmental Science and Technology, Jessore University of Science & Technology, Bangladesh
Correspondence: Kishor Mazumder, School of Biomedical Sciences Charles Sturt University Boorooma St, Locked Bag 588 Wagga Wagga, NSW 2678, Australia
Received: September 18, 2018 | Published: October 29, 2018
Citation: Mazumder K, Dey ZK, Dey S, et al. Phytochemical screening &evaluation of ant diarrheal mode of action of leaves extracts of Paederia foetida linn. Int J Complement Alt Med. 2018;11(5):304-309. DOI: 10.15406/ijcam.2018.11.00416
For medicinal purpose, decoction of the whole plant P. foetida or leaf, stem bark juice are prepared. Tender leaves are boiled and eaten with chili and salt as food. Tribal people of Chittagong hill tracts of Bangladesh and Aka tribe of Arunachal Pradesh, India consume the leaf juice to treat diarrhea, dysentery, and burns or scalding. It has also been reported that, the powder form of the whole plant is taken by certain tribal communities in India for weakness and rheumatic joint pains. Though the plant has traditional usage as an anti diarrheal, there were limited studies to evaluate its anti diarrheal mode of action. Current study evaluates the preliminary phytochemical screening and anti diarrheal mode of action of aqueous & methanolic extracts of leaves of P. foetida. Preliminary phytochemical study revealed the presence of steroid, alkaloid, saponin, flavonoid, phenolic acids and tannin in the leaves extracts. Swiss albino mice of either sex were divided into six groups (five/group): Group-I served as control and received vehicle (0.9% NaCl-saline) at a dose of 2 ml/kg orally; Group-II served as standard and received loperamide at the dose of 5mg/kg i.p; Group-III and IV received aqueous extract of Paederia foetida, whereas group-V and VI received ethyl-acetate extract of Paederia foetida at doses of 200 and 300mg/kg i.p, respectively. Castor-oil induced diarrhea, castor-oil induced enteropooling and gastrointestinal motility test were performed to investigate anti diarrheal activity of these extracts at given groups and concentrations. Acute-toxicity test was also performed at four different doses. The diarrheal episode was inhibited by 39.68% and 49.21% for aqueous extract whereas 47.62% and 53.97% for ethyl acetate extract at the doses of 200 and 300mg/kg respectively. The aqueous extract significantly (p<0.05) decreased the intestinal volume (0.48±0.04ml for 300mg/kg). Ethyl acetate extract also shown the similar result (0.47±0.027ml for 300mg/kg) for the same dose compared to control (0.65±0.03ml) in castor-oil induced enter opooling. Furthermore, decreased intestinal transit (57.1–62.34%) were observed for aqueous extract whereas (59.43–63.50%) for ethyl acetate extract compared to standard (loperamide 5mg/kg). No delayed toxicity was also observed for both of these extracts. Though both the extracts showed potential antidiarrheal activity however the ethyl acetate extract showed more significant activity than the aqueous extract of Paederia foetida. The mode of anti diarrheal activity is due to both the inhibition of gastrointestinal motility and enteropooling activities.
Keywords: anti diarrheal, Paederia foetida, castor oil, intestinal transit, acute-toxicity
In developing countries, diarrhea is a major health problem. Acute diarrhea is the leading cause of morbidity and mortality amongst children in developing countries,1 Diarrhea occurs due to a series of imbalanced physiological steps such as increasing motility of the gastrointestinal tract, increased secretion of ions and a decrease in the absorption of fluid and as a consequence loss of electrolytes, particularly Na+ and water.2 Diarrhea has long been recognized as one of the most important health problem in the developing countries. Worldwide, diarrhea accounts for more than 5-8million deaths in infants and small children less than 5years each year. According to World Health Organization (WHO) estimation for the year 1998, there were about 7.1million deaths due to diarrhea.3 In the developing countries, diarrheal disease caused 5.2% of overall deaths and became the sixth leading cause of death in the year of 2007.4 Diarrhea causes one third of the child death in Bangladesh.5 In this scenario, WHO proposed the use of traditional plant in its Diarrhea Control Program.6
Paederia foetida (Bengali name- Gondhabadali, Gondhal; English name- King’s Tonic) is a climbing twining shrub belonging to the family Rubiaceae and found all over the Bangladesh.7,8 Leaf juice of the plant acts as astringent and is given to children in diarrhea. The leaves extract shows diuretic activity whereas, the roots and barks are widely used to induce nausea and vomiting and in the treatment of hemorrhoids, inflammation of spleen and chest pain and liver pain. Fruits of P. foetida is specifically used against toothache.9 The methanolic extract of P. foetida (leaves) showed significant results on the investigation of anti-microbial, cytotoxic and anti-helmentic activity.10,11 Whether, ethanolic extract of leaves showed anti-bacterial and hepatoprotective activity.12,13 The root parts of P. foetida help to treat gastric-ulcer.14 On the other hand, the whole plant exerts anti-nociceptive activity. Phytochemical screening showed that arial parts contain a methyl mercaptane, crystalline keto alcohol, paederolone, aketo compound, paederone and beta-sitosterol,protein, carbohydrates, glucosides essential oils, quinines, alkaloids, iridoid glycosides, sitosterol, stigmasterol, amino acid and volatile oil etc.15‒17 Though the plant has been used traditionally as a remedy for diarrhea and dysentery in many parts of Bangladesh for long time, a few studies have reported. Afroz et al.,18 reported that ethanolic extract of P. foetida have potential Antidiarrheal activity on limited mice models for ant diarrheal and gastrointestinal motility. Haider et al.,19 performed a randomized double blind clinical trial of the dried plant extract against a small group of patients with acute shigellosis and found moderate effect in comparison to ampicillin. In addition, recently Swarnamoni et al.,20 reported that ethanolic leaves extracts exhibited significant amelioration of acetic acid induced colitis in mice model.
Therefore, all of the previous study justified the present work for more and in depth investigations of the antidiarrheal activities of the leaves of the plants in different animal models of various extracts from different solvents such as aqueous and ethyl-acetate to explore the antidiarrheal mode of action along with acute toxicity to get in site the mechanism of antidiarrheal action as well as the safety of the plant extracts.
Plant material
The selected plant (Paederia foetida) was collected from a local area (Colonel Hat) of Chittagong district, Bangladesh and authenticated by Dr. Shaikh Bokhtear Uddin, Chittagong University Herbarium, Department of Botany, University of Chittagong, Chittagong, Bangladesh (accession no. SBU 2371. Dt. 11.05.2014. CTGUH).
Preparation of extract
The leaves were shade dried and ground. The ground leaves (300g for each solvent) were soaked in sufficient amount of distilled water and ethyl acetate for two weeks at room temperature with occasional shaking and stirring. In case of aqueous extraction a small amount of sodium azide is added as a preservative. After that both the extracts were filtered through a cotton plug followed by Whitman filter paper No. 1. The mixer was vacuum dried at room temperature to yield semisolid. The extract was then preserved in a refrigerator till further use.
Animals
Swiss Albino mice of either sex, weighting 25 to 30gm was obtained from animal house of Department of Pharmacy, Jahangirnagar University, Dhaka, Bangladesh and housed in polypropylene cages under controlled conditions. The animals were exposed to alternative 12h light/12h dark cycle at an ambient temperature of 27±1ºC with a relative humidity of 55%-65%. Animals were allowed free access to drinking water ad libitum and pellet diet (obtained from International Centre for Diarrheal Disease and Research, Bangladesh). All the animals were acclimatized for 10days in the laboratory environment prior to the study. For conducting all experiments with animals, we followed the guidelines of Institutional Animals Ethics Committee (IAEC) and before the experiments, study protocols were approved by the University of Science and Technology Medical Ethics, Biosafety and Biosecurity Committee (USTMEBBC) at the Life Science Faculty, University of Science and Technology Chittagong, Bangladesh.
Chemicals
Following chemicals were used for evaluation of ant diarrheal activity of P. foetida
Phytochemical screening
Both the extracts of P. foetida were evaluated by phytochemical qualitative reactions for usual plant secondary metabolites. The phytochemical screening was conducted for the identification of steroids, alkaloids, coumarins, flavonoids, saponins, tannins and phenolic acids.21‒24
Following reagents and chemicals were used for phytochemical screenings of the extracts: Dragendroff’s reagents (for alkaloids),mg and HCl (for flavonoids); Ferric chloride and Potassium dichromate solutions (for tannins) and saponins with ability to produce stable foam and Libermann- Burchard reagent (for steroids). Phenolic acids derivatives were identified in the studied of water extract of P. foetida leaves by the Carrez reagent.
Acute toxicity test
We utilized twenty Swiss Albino mice (4-5weeks old, weighing 20-25g) for investigating acute toxicity and with this we estimated LD50 value. Both the aqueous and ethyl-acetate extract was dissolved in saline water and administered orally to four groups of mice, each group containing five mice, at different doses (200, 400, 1000 and 2000mg/kg, b/w). After 24hours, LD50 was evaluated by recording mortality.25
Ant diarrheal Activity
Castor oil induced diarrhea
We followed the method of Awouters et al to evaluate the antidiarrheal activity of Paederia foetida.26 The experimental mice were fasted 18h with water ad libitum before experiment. We divided the mice in six groups with each group containing five mice. The groups were marked as- Group I (Control group: treated with saline 2ml/kg body weight orally), Group II (Standard group: treated with loperamide 5mg/kg b. wt. i.p), Group III-IV (treated with 200 and 300mg/kg b. wt. dose of P. foetida aqueous extract i.p.) and Group V-VI (treated with 200 and 300mg/kg b. wt. dose of P. foetida ethyl acetate extract i.p.). After 1hour interval, each animal was given castor oil orally in the purpose of inducing diarrhea. Then the mice were caged with white blotting paper placed at the bottom of the cage and the papers were changed every 1hour for the purpose of ease in stool count. The total number of both dry and wet feces was counted every hour and the process continued up to 4hours. Then the total number of excreted feces was compared with control group whereas total number of diarrheal feces of the control group was considered 100%.26
Castor oil induced enteropooling
For experimenting castor oil induced enter pooling, we followed the method of Robert et al.27 The animals were fasted for 18 hours prior to experiment with water ad libitum and the fasted mice were grouped as Group I (Control group: treated with saline 2ml/kg body weight orally), Group II (Standard group: treated with loperamide 5mg/kg b. wt. i.p), Group III-IV (treated with 200 and 300mg/kg b. wt. dose of P. foetida aqueous extract i.p.) and Group V-VI (treated with 200 and 300mg/kg b. wt. dose of P. foetida ethyl acetate extract i.p. with each group containing five animals. After 1 hour, the animals were given castor oil orally for inducing diarrhea. The with 1hour interval each animal was sacrificed with over dose of chloroform anesthesia and the sacrificed mice were dissected to separate the small intestine from pyloric sphincter to ileocecal junction. The intestine was weighed and the contents were milked out with graduated tube and volume of the contents was measured. Then the intestines were reweighed and weight gap between full and empty stomach were calculated.28
Gastrointestinal motility test
We carried out the experiment of gastrointestinal motility test according to the method described by Mascolo et al.29 The animals were fasted for 18hours prior to experiment with water ad libitum and the fasted mice were grouped as- Group I (Control group: treated with saline 2ml/kg body weight orally), Group II (Standard group: treated with loperamide 5mg/kg b. wt. i.p), Group III-IV (treated with 200 and 300mg/kg b. wt. dose of P. foetidaaqueous extract i.p.) and Group V-VI (treated with 200 and 300mg/kg b. wt. dose of P. foetida ethyl acetate extract i.p. with five animals in each group. Before 1hour of treating the groups with respective materials, the animals were given castor oil orally to induce diarrhea. After 1hour of treating the animals with respective material, the animals were given charcoal preparation (10% charcoal suspension in 5% gum acacia) orally for tracking the movement of the contents afterwards. With one hour interval, the animals were sacrificed by overdose of chloroform anesthesia and the distance traveled by the charcoal preparation from pylorus to caecum was measured and expressed as a percentage of the total distance of the intestine.
Statistical analysis
All results are expressed as (mean±standard error) and to represent the significance, one-way ANOVA (Analysis of Variance) followed by Bonferroni test were used. P value less than 0.05 or 0.001 was considered statistically significant. All data were calculated with SPSS software (version: 16; IBM corporation, New York, USA).
Phytochemical screening
Preliminary phytochemical screening revealed that different components such as steroid, tannins, alkaloids and phenolic acids are present in the extract which is demonstrated in Table 1.
Extracts |
Steroids |
Alkaloids |
Coumarins |
Flavonoids |
Saponins |
Tannins |
Phenolic acids |
PFA |
- |
++ |
- |
++ |
++ |
+++ |
++ |
PFEA |
+ |
+ |
- |
++ |
- |
++ |
- |
Table 1 Result of chemical group test of different extracts of P. foetida
* PFA, P.foetida aqueous extract, PFEA, P.foetida ethyl acetate extract
* [+++, Strong intensity reaction, ++, Medium intensity reaction, +, Weak intensity reaction, −, not detected]
Acute toxicity test
For the LD50 dose determination, aqueous and ethyl-acetate extract of P. foetida leaves were administered up to dose 2000mg/kg body weight. In the 14days monitoring period, we did not observe any visible signs of acute toxicity and mortality.
Castor oil induced diarrhea
In the castor oil-induced diarrhea experiment, the leaves extract of P. foetida produced a marked ant diarrheal effect in the mice (Table 2). At doses of 200 and 300mg/kg, the aqueous and ethylacetate extract displayed significant (p<0.001) defecation compared to control group. The decreased number of excreted feces after administration of castor oil, for aqueous extract the values are 7.20±0.37(200mg/kg) and 6.40±0.51 (300mg/kg), whereas, for ethyle acetate extract the values are 7.20±0.37 (200mg/kg) and 6.40 ±0.51 (300mg/kg). On the other hand, for loperamide, the number of feces decreased to 4.80±0.37 at the dose of 5mg/kg compared to control group (Figure 1).
Groups |
Treatment |
Total number of feces |
% Inhibition of defecation |
Total number of diarrheal feces |
% Inhibition of diarrhea |
|
Control |
I |
Castor oil + Saline |
15.4±0.51 |
--- |
12.60±0.51 |
-- |
Positive |
II |
Castor oil +Loperamide |
7.60±0.51** |
50.67 |
4.80±0.37** |
61.90 |
P. foetida (Aqueous extract) |
III |
Castor oil + Extract (200mg/kg i.p) |
11.8±0.37** |
19.48 |
7.20±0.37** |
39.68 |
IV |
Castor oil + Extract (300mg/kg i.p) |
10.0±0.71** |
35.06 |
6.40±0.51** |
49.21 |
|
P. foetida (Ethyl Acetate extract) |
V |
Castor oil + Extract (200mg/kg i.p) |
12.2±0.74* |
20.78 |
6.60±0.51** |
47.62 |
VI |
Castor oil + Extract (300mg/kg i.p) |
11.6±0.51* |
24.68 |
5.80±0.40** |
53.97 |
|
Table 2 Effect of aqueous and ethyl acetate-extract of P. foetida on castor-oil induced diarrhea in mice
*[Values are expressed as mean±SEM (n=5). *p<0.05, **p<0.001 when compared with control group]
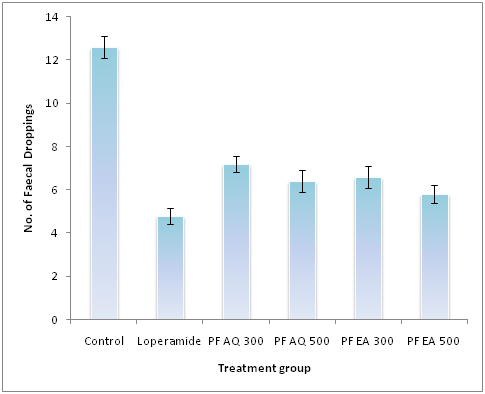
Figure 1 N=57; Epidemiological distribution of the pathological fractures, traumatic fractures, and nonunion.
Castor oil induced enteropooling
In case of Castor oil induced enteropooling test, the P. foetida extracts (200 and 300mg/kg) produced a significant reduction in intestinal weight and volume in a dose dependent manner (Table 3). The intestinal volume was decreased by 17.35% (200mg/kg) and 26.02% (300mg/kg) for aqueous extract whereas for ethyl-acetate extract it was 21.43% (200mg/kg) and 27.55% (300mg/kg). The standard drug, loperamide (5mg/kg), also significantly inhibited (p<0.05) intestinal fluid accumulation which is 37.75% compared to control group.
Groups |
Treatment |
Weight of intestinal content(g) |
Volume of intestinal content(mL) |
(%) Inhibition |
|
Control |
I |
Castor oil + Saline |
1.75±0.08 |
0.65±0.03 |
-- |
Positive |
II |
Castor oil + Loperamide |
1.50±0.03 |
0.41±0.01* |
37.75 |
P. foetida (Aqueous extract) |
III |
Castor oil + Extract |
1.69±0.05 |
0.54±0.25 |
17.35 |
IV |
Castor oil + Extract |
1.55±0.05 |
0.48±0.04* |
26.02 |
|
P. foetida |
V |
Castor oil + Extract |
1.72±0.03 |
0.51±0.02 |
21.43 |
VI |
Castor oil + Extract |
1.57±0.03 |
0.47±0.02* |
27.55 |
|
Table 3 Effect of aqueous and ethyl-acetate extract of P. foetida on castor-oil induced enteropooling in mice
*[Values are expressed as mean±SEM (n=5). *p < 0.05, **p<0.001 when compared with control group]
Gastrointestinal motility test
Both the extracts of P. foetida significantly (p<0.001) reduced the gastrointestinal transit. In case of the aqueous extract these were 22.33±1.20cm (51.1%) for 200mg/kg and 19.33± 1.20 cm (62.34%) for 300mg/kg respectively. Whereas, in case of ethyl acetate extract these were 21.42±0.87cm (59.43%) for 200mg/kg and 19.26±1.20cm (63.50%) for 300mg/kg respectively. These values clearly revealed that both extracts decreased travel of the charcoal preparation while compared to the control (40.67±3.48cm) group by inhibition of GI motility (Table 4). However, lope amide (5mg/kg) also produced a significant decrease 14.66 ±1.45cm (72.18%) in the propulsion of charcoal preparation through gastrointestinal tract.
Groups |
Treatment |
Total length of intestine (cm) |
Distance travelled by marker (cm) |
Inhibition (%) |
|
Control |
I |
Castor oil+Saline |
54.00±0.58 |
40.67±3.48 |
-- |
Positive |
II |
Castor oil+Loperamide (5mg/kg i.p) |
52.70±0.88 |
14.66±1.45** |
72.18 |
P. foetida (Aqueous |
III |
Castor oil+Extract |
52.0±1.15 |
22.33±1.20** |
57.1 |
IV |
Castor oil+Extract |
51.33±0.88 |
19.33±1.20** |
62.34 |
|
P. foetida (Ethyl acetate extract) |
V |
Castor oil+Extract |
52.80±0.44 |
21.42±0.87** |
59.43 |
VI |
Castor oil+Extract |
52.77±0.77 |
19.26±1.20** |
63.50 |
|
Table 4 Effect of aqueous and ethyl-acetate extract of P. foetida on small-intestinal transit in mice
*[Values are expressed as mean±SEM (n=5). * p<0.05, ** p<0.001 when compared with control group]
As part of the traditional therapy, many people from different regions of the world use medicinal plants. Not only whole plant but also some specific parts (such as leaves, bark, etc.) of the plant used as the herbal therapy. As a part of traditional use, leaves of Paederia foetida also used for the treatment of diarrhea in some hilly regions of Bangladesh. We found through our preliminary phytochemical screening thatboth aqueous and ethyl acetate extract of P. foetida (leaves) possess some compounds like alkaloid, tannin, flavonoids, steroids, saponin etc. which may be responsible for amelioration of diarrhea.
The aqueous and ethylacetate extracts of P. foetida (leaves) displayed the significant antidirrheal activity (p<0.001) at 200mg/kg and 300mg/kg doses which is comparable to the standard drug loperamide (5mg/kg) and also possess 19.48% and 35.06% inhibition of defecation for the aqueous extract while 20.78% and 24.68% inhibition for the ethyl acetate extract respectively. On the other hand, in case of Castrol oil induced enteropooling, both the extracts displayed significant (p<0.05) effect at 300mg/kg dose as well as reduced the intra-luminal contents volume. These effects, which have direct consequences to reduce water and electrolytes secretion into the small intestine,30 suggested that the extracts may enhance electrolyte absorption from the intestinal lumen consistent with inhibition of hyper-secretion. Hyper-motility characterizes diarrhea where the secretory component is not the causal factor.31 Pre-treatment with both the extracts reduced the peristaltic movement or transit of charcoal preparation through the gastrointestinal tract which significantly (p<0.001) indicates that it may be feasible to reduce the frequency of stooling in diarrheal conditions with the leaves extract of P. foetida such as 57.1% and 62.34% inhibition by aqueous extracts whereas 59.43% and 63.50% inhibited by ethylacetate extract at a respective dose of 200mg/kg and 300mg/kg.
Castor oil induces diarrhea by following mechanisms-inhibition of intestinal Na+ K+ ATPase activity, thus reducing normal fluid absorption,32 activation of adenylate cyclase or mucosal cAMP-mediated active secretion,33 stimulation of prostaglandin formation and platelet activating factor.34 In the recent time, it is also proposed that nitric oxide also plays an important role in diarrheal effect of castor oil. However, it is an established concept that a compound named ricinoleic acid found in castor oil is responsible for inducing diarrhea for its hyper secretory activity.35,36 Above observations suggested that both the extracts reduced diarrhea by inhibiting peristalsis, gastrointestinal motility and castor oil induced enter pooling in a dose dependent manner. Earlier studies proved that tannins, alkaloids, flavonoids, sterol and/or triterpenes are responsible for anti-dysenteric and antidiarrheal properties of medicinal plants.37‒39 Hence, phytochemical screening confirmed the presence of tannins, alkaloids and sterol in the leaves of the plant so these bio active compounds might be responsible for the mechanism of action of P. foetida ant diarrheal activity.
The results of this investigation revealed that the aqueous and ethyl acetate extract of P. foetida leaves possess significant anti diarrheal properties. The mechanism of antidiarrheal activity of the plant is due to the significant inhibition of gastrointestinal motility and enteropooling activities. Advanced research is needed to isolate specific compounds from both aqueous and ethyl acetate extracts and find out the specific molecule(s) responsible for the observed anti diarrheal activity.
Authors are thankful to Dr. Shaikh Bokhtear Uddin, Associate Professor, Department of Botany, and University of Chittagong, Bangladesh who helped to authenticate the plant. Authors also gratefully acknowledge the Department of Pharmacy, Jahangirnagar University, Dhaka, Bangladesh for supplying animals.
There is no conflict of interest associated with the authors of this paper.

©2018 Mazumder, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.