International Journal of
eISSN: 2381-1803


Research Article Volume 14 Issue 6
1Department of Immunology, Allergy Midwest Allergy Sinus Asthma PC, USA
1Department of Immunology, Allergy Midwest Allergy Sinus Asthma PC, USA
Correspondence: Robert Kaufmann, Department of Immunology, Allergy Midwest Allergy Sinus Asthma PC, Director of Research, Shaman Botanicals LLC, USA
Received: October 02, 2021 | Published: November 2, 2021
Citation: Kaufmann R. Nano-processed CBG/CBD effect on pain, adult attention deficit hyperactive disorder, irritable bowel syndrome and chronic fatigue syndrome. Int J Complement Alt Med. 2021;14(6):235-240. DOI: 10.15406/ijcam.2021.14.00567
Objective: Cannabigerol (CBG) and Cannabidiol (CBD) have similar anti-inflammatory and analgesic effects but different mechanisms of action. A preliminary trial of a novel nano-processed combination of CBG and CBD seemed to be effective in irritable bowel disease but was also reported to increase energy, thinking, attention, calmness, and sleep while reducing fatigue and anxiety. This survey was designed to determine if the same findings were reported by a larger group of individuals.
Methods: Adult users (>21years of age) of this unique form of CBG/CBD were asked to complete an online survey of their experience after a minimum of seven days of use. The survey asked questions about fatigue, energy, thinking, attention, calmness, sleep, and anxiety and about the products effect on pain, attention deficit and hyperactivity disorder (ADHD), chronic fatigue syndrome (CFS), irritable bowel syndrome (IBS), ulcerative colitis, and Crohn’s disease.
Results: A total of 220 subjects completed the survey. Over 70% of the respondents reported the product improved their energy and attention levels and their thinking and decreased their fatigue levels. Calmness and anxiety were improved in 63.2% and 58.6% of the respondents, respectively, while only 44.1% reported sleep improvements. No one reported any adverse effects. Of the respondents that reported pain, 57.6% reported improvements in their pain, with 51.2% being able to reduce or stop their pain medications. 70.8% of the respondents with ADHD, 83.3% of those with chronic fatigue, and 62.1% of those with IBS all reported improvements in their conditions.
Conclusions: This nano-processed CBG/CBD product improved ADHD, chronic fatigue, and IBS symptoms and relieved pain, such that over half of individuals were able to decrease or stop their pain medication. In addition, it decreased fatigue, improved energy and thinking, improved attention levels, and may have a positive effect on anxiety, calmness, and sleep.
Keywords: irritable bowel syndrome, hyperactive disorder, chronic fatigue, chronic fatigue, cannabigerol, nanotechnology, ulcerative colitis, anxiety
CBG, cannabigerol; CBD, cannabidiol; ADHD, attention deficit and hyperactivity disorder; CSF, chronic fatigue syndrome; IBS, irritable bowel syndrome; UC, ulcerative colitis
Cannabigerol (CBG) and cannabidiol (CBD) have been shown to have several anti-inflammatory effects, including the following: reduced inflammatory cytokines, increased anti-inflammatory cytokines, reduced nitric oxide production by macrophages, reduced reactive oxygen species, augmented anti-oxidant defenses, and decreased oxidative stress.1-5 Their effect on specific inflammatory compounds is variably similar, different, or additive, causing their effects on other tissues to elicit similar or different responses that vary in intensity.6,7 This is thought to be due to variation in their affinity and attachment to receptors, which then affects their efficacy and potency on specific receptors.8-12
A formulation with a 3:1 ratio of CBG and CBD treated with nanotechnology was tested on a small number of individuals who reported it was effective in reducing pain and inflammation, especially in individuals with inflammatory and/or irritable bowel disease. An unexpected finding was that most individuals also reported improvements in their energy level, thinking, attention level, and sleep. In addition, they reported experiencing less fatigue, felt calmer, and had less anxiety. Therefore, a survey was conducted to determine if a larger number of individuals taking this product had similar experiences and if any adverse side effects were experienced.
The formulation used for this study combined 15mg/ml of CBG and 5mg/ml of CBD treated with nanotechnology to improve its absorption and to make it water soluble. While the recommended serving is 0.5ml, individuals often find that they need to vary this amount to achieve the maximum effect, as is the case with all cannabinoids. Individuals took more or less per serving as needed to obtain their desired effect. Adult users (>21years of age) of this unique form of CBG/CBD were asked to complete a survey of their experience after a minimum of seven days of use. Individuals were given a brochure when they purchased the product that explained the survey and how to access it, if they desired.
The survey was conducted online using a proprietary program. The survey asked about dosing and frequency of use, as well as age, sex, height and weight. The survey had two sections: Section 1 asked questions on energy level, thinking ability, attention level, level of calmness, sleep quality, anxiety level and fatigue level. Available answers were: much worse, worse, same, improved, and much improved, or a variation thereof that was appropriate for the question, which were given intensity score from -2 to +2, respectively. Section 2 asked about pre-existing conditions – such as pain, attention deficit and hyperactivity disorder (ADHD), chronic fatigue syndrome (CFS), irritable bowel syndrome (IBS), ulcerative colitis (UC), and Crohn’s disease – and about any unexpected and/or adverse side effects. Similarly, for each of the conditions, they were asked if their overall condition was much worse, worse, same, improved, and much improved and intensity scores were assigned from -2 to +2, respectively. Subjects with pain who answered that their pain had improved or much improved were asked if they had been able to make any changes to their pain medication, that is, whether they had:
Subjects with ADHD were asked three additional questions about their attention level, hyperactivity, and impulsiveness which had possible answers of much worse, worse, same, improved, and much improved, to which intensity scores from -2 to +2 were assigned, respectively.
To determine if positive responses were due solely because of a placebo effect, a value of 45% of individuals having positive responses was used as the hypothesized placebo population response and a one proportion z-test was performed on all intensity levels.13
A total of 220 subjects completed all questions. The demographics of the respondents are presented in Table 1. Almost twice as many female (114) as males (76) responded. Significant differences existed between females and males in age, height, and weight with males being younger, taller, and heavier (Table 1). However, no differences in BIM, individual dose amount and total dose per day between the sexes (Table 2).
|
Gender |
Totals |
Age (yrs) |
Height (in) |
Weight (lb) |
||||||
|
Mean |
S.D |
Range |
Mean |
S.D |
Range |
Mean |
S.D |
Range |
||
|
Overall |
220 |
44.3 |
14.7 |
19-77 |
66.4 |
4.3 |
53-79 |
174 |
47.8 |
98-330 |
|
Male |
76 (34.5%) |
39.7 |
13.8 |
20-65 |
70.3 |
2.8 |
64-79 |
191.1 |
39.7 |
125-310 |
|
Female |
144 (65.5%) |
46.7 |
14.7 |
19-77 |
64.3 |
3.4 |
53-71 |
165.2 |
49.6 |
98-330 |
|
p-values |
<0.001 |
<0.001 |
<0.001 |
|||||||
Table 1 Demographics are presented for the sexes and overall
|
Gender |
Totals |
BMI |
Individual Dose (ml) |
Total Daily Dose (ml) |
||||||
|
Mean |
S.D |
Range |
Mean |
S.D |
Range |
Mean |
S.D |
Range |
||
|
Overall |
220 |
27.8 |
7.4 |
14.5-56.6 |
0.69 |
0.28 |
0.25-1.25 |
0.96 |
0.80 |
0.25-7.00 |
|
Male |
76 (34.5%) |
27.1 |
5.2 |
18.4-43.1 |
0.68 |
0.27 |
0.25-1.00 |
0.84 |
0.48 |
0.25-2.00 |
|
Female |
144 (65.5%) |
28.1 |
8.4 |
14.5-56.6 |
0.70 |
0.70 |
0.25-1.25 |
1.03 |
0.93 |
0.25-7.00 |
|
p-values |
n.s. |
n.s. |
n.s. |
|||||||
Table 2 BMI and individual dose and total daily dose amount of CBN for the sexes and overall
General feelings
The respondents’ evaluation of CBG on fatigue level, energy level, thinking ability, and attention level are listed in Figure 1. All four of these conditions had >70% of the intensity scores being positive. Energy was rated as improved (+1 intensity) or much improved (+2 intensity) by 76.4% of the respondents while 74.1% reported positive intensity scores of less fatigued (+1 intensity) or much less fatigued (+2 intensity), and thinking clarity and attention level had 72.9% and 71.4% positive intensity scores, respectively. Level of calmness and anxiety level positive intensity scores were less at 63.2% and 58.6% respectively, however, only 44.1% of the subjects had a positive intensity score for sleep quality (Figure 2). A z-test result yielded a p-value of <0.001 for all intensity scores, except for sleep quality (p 0.39).
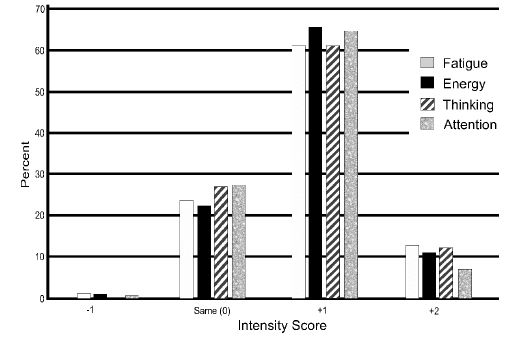
Figure 1 The magnitude of change from before using CBG to after using CBG is presented for fatigue, energy level, thinking ability, and attention are presented. The magnitude of change is quantified from -2 to +2 from much worse to much improved, respectively.
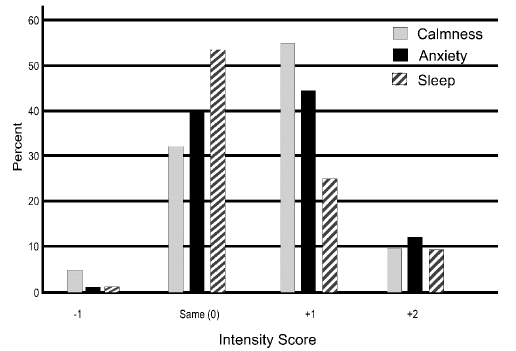
Figure 2 The magnitude of change from before using CBG to after using CBG is presented for level of calmness, anxiety level, and sleep quality.
Pre-existing conditions
Pain: Of the 220 respondents, 118 (53.6%) reported that they had been suffering from pain. One of the 118 respondents reported that their pain was worse, but 68 (57.6%) of them reported a reduction in pain with 48 (40.7%) reporting improved pain and 20 (16.9%) reporting much improved pain (p-value= 0.003). Of those with less pain, 51.2% reported taking less pain medication(p=0.152), with 10.7% reducing their pain medications by half, 9.1% almost stopping their pain medications, and 9.9% completely stopping their pain medications (Figure 3).

Figure 3 The amount of reduction in pain medication usage in individuals with pain after they started using CBG.
ADHD: Forty-three (19.5%) of the 220 respondents reported that they had been diagnosed with ADHD. Of these respondents, 90.7% reported an improvement in their attention level – much improved, 11.6%; improved, 79.1% – while no one reported worsening of attention (p,0.001). Similarly, 63.5% (p=0.007) reported improvement in hyperactivity (51.2% improved and 2.3% much improved), while 62.8% (p=0.009) reported improvement in Impulsiveness (53.5% improved and 9.3% much improved) (Figure 4). Chi-squares analysis comparing those with ADHD to those without found the ADHD subjects reported significantly more improvement in attention (p<0.05), anxiety (p < 0.005), and calmness (p < 0.001).

Figure 4 The magnitude of change from before using CBG to after using CBG is presented for attention, hyperactivity, and impulsiveness for those individuals having ADHD.
CFS: Fifty (22.7%) of the respondents reported they suffered from CFS. Of these, 78.0% reported an improvement in their condition (p<0.001) – much improved: 18.0%; improved: 60.0% – and none reported their condition was worse while on this product (Figure 5).
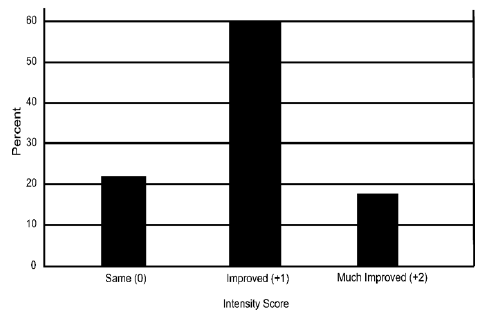
Figure 5 The magnitude of change from before using CBG to after using CBG is presented for individuals suffering from Chronic Fatigue Syndrome (CFS).
IBS: Forty-six of the respondents (20.9%) reported that they suffered from IBS. Of these, 56.5% (p=0.058) reported their condition was improved (47.8%) or much improved (8.7%) with this product, while only one individual reported their condition as worse (Figure 6). Additionally, 39 (84.7%) of these respondents with IBS reported that they had pain, and 61.5% reported less pain (p=0.019) – much less: 17.%; less: 4.6% – and no one reported their pain was worse. Of these IBS patients with pain, 51.3% were able to reduce their use of pain medication (p=0.214) with 15.4% being able to reduce their pain medication by half or almost stop their pain medication and 7.7% being able to completely stop their pain medication.
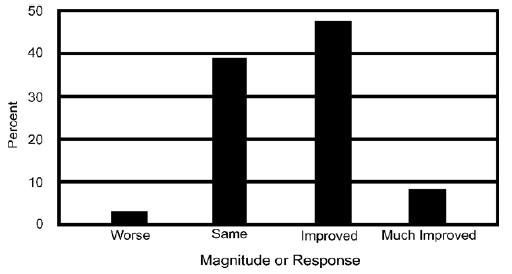
Figure 6 The magnitude of change from before using CBG to after using CBG is presented for individuals suffering from Irritable Bowel Syndrome (IBS).
Other inflammatory bowel disease: Only four individuals stated they suffered from UC, and one of these three felt the UC was improved on this product. No one reported that they suffered from Crohn’s disease.
CBD is well known to decrease anxiety and produce a feeling of calmness and possibly improve sleep.14-16 However, this is the first time that CBG and/or CBD has been reported to increase energy and attention levels, decrease fatigue, and improve thinking. Whether this is due to the combination of CBG and CBD in this product or due to the nano processing is unknown; but the number of individuals reporting these effects and the magnitude of the effects are striking. The amount of CBD taken by the individuals in this survey (average < 5 mg of CBD) is very low suggesting that that majority of the effect may be due to CBG. Although a nano processed CBD product taken in this dose range has been shown to often decrease anxiety and promote calmness, it is unlikely that this low amount of CBD alone would produce a positive effect on sleep.17
Over half of the subjects with pain reported pain reduction and reduction in use of pain medications with use of this nano-processed CBG/CBD product. The magnitude of the reduction was consistent across all the diseases reported in this survey. Whether this was due to an effect on inflammation, a direct effect on pain receptors, or a decrease in pain perception cannot be determined by this survey. However, animal studies have shown that CBD and CBG have been shown to decrease inflammation, that CBD and CBG bind to peripheral neural receptors, and that CBD has a central analgesic action similar to morphine.1–5,20,21 However, CBD’s and CBG’s binding to the receptors involved with inflammation and on neurons are different, suggesting that they may have synergistic or entourage effects.1-5
In addition, this is the first report of CBG and/or CBD improving IBS. IBS causes pain and has a significant negative effect on quality of life.20 CBG and CBD have been studied in animal models of inflammatory bowel disease and both have been found to be beneficial.2,21-24 CBD has been studied in humans with inflammatory bowel disease and found to improve quality of life but failed to reduce inflammatory markers.23 Yet two reviews of the Cochrane library found that the efficacy and safety of CBD in inflammatory bowel disease is still uncertain.25,26 Irritable bowel syndrome pathogenesis involves inflammation in the bowel, but it also involves increased epithelial hyperpermeability, dysbiosis, visceral hypersensitivity, epigenetics and genetics, and altered brain–gut interactions.27,28 CBG and CBD have been shown to decrease pain in humans with gut inflammation.21,29 Although the mechanism by which this occurs is unknown, animal studies suggest that it may include most, if not all, of the typical pathogenic alterations, as well as reducing the perception of pain in the brain.21-23,27-30 Whether the improvement in IBS with this product is due to its effect on inflammation or one or more of these other factors is not known.
In survey studies and studies of pain, the placebo effect can vary significantly from 10-45%.13 This study was a survey of present users and may include significant bias, in that users who had not experienced significant benefit may have not filled out the survey, therefore the highest percentage for placebo effect (45%) was used. However, the magnitude of the improvement seen in this survey of 220 individuals was so large that the observed proportions of positive effect were significant for almost all conditions evaluated, suggesting that the effect being seen is not due solely to placebo.
The nano processed CBG/CBD product used in this study was found to relieve pain and irritable bowel syndrome symptoms, enabling up to 60% of individuals to decrease or stop their pain medication. In addition, it decreased fatigue, improved energy and thinking, improved attention levels, and may have some influence on anxiety, calmness, and sleep. These effects seemed to be particularly useful for those with ADHD, especially in the aspects of improved thinking and attention. The magnitude of the positive effect of this product was so large that the likelihood that it was all due to placebo effect is almost entirely eliminated. Therefore, the findings of this survey warrant more definitive studies of this product in individuals suffering from these conditions to determine the true magnitude of this effect.
None.
Dr. Kaufmann is Director of Research for Shaman Botanicals, LLC declares no conflicts of interest.
No funding sources.

©2021 Kaufmann. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.