International Journal of
eISSN: 2381-1803


Ethnopharmacological relevance: Mimosa caesalpiniifolia popularly known as “sabiá” or “sansão-do-campo” is a Brazilian Northeast native perennial tree used for several purposes and in traditional medicine is used for inflammatory diseases, hypertension and fungal infections.
Aim of the study: The objective of this study was to identify the compounds in the hydroalcoholic extract of EEM leaves encapsulated with aerosil® 20%, and to evaluate their antioxidant and anti-inflammatory.
Material and methods: The antioxidant activity of EEM leaf hydroalcoholic extract was determined by using both the 1,1-diphenyl-2-picrylhydrazyl radical scavenging and the oxygen radical absorbance capacity in vitro assay. The phytochemical study of EEM was analyzed by experiments with FIA-ESI-IT-MSn (Direct Flow Analysis-ionization Electrospray Ion Trap Tandem Mass Spectrometry). Anti-inflammatory properties of EEM were evaluated in ear edema induced by xylene in rats.
Results: The phytochemical investigation of EEM resulted in the identification of flavonoids glycosides (m/z 563, 579, 621) and derivatives of catechin (m/z 595, 611, 741 and 757). EEM leaf extract had antioxidant and anti-inflammatory properties. EEM (125 and 250mg/kg, p.o.) significantly inhibited the ear edema induced by xylene (52% and 64% respectively).
Conclusion: Our results suggest the antioxidant action and the anti-inflammatory effect in the mouse ear edema model of EEM are related to the presence of flavonoids and catechin derivatives revealed in phytochemical screening. These results support the ethnopharmacological use of Mimosa caesalpiniifolia in folk medicine.
Keywords: Mimosa caesalpiniifolia, flavonoids, anti-inflammatory, antioxidant, hydroalcoholic extract
Mimosa is one of the largest genera of Mimosoid legumes, native From South America and comprises about 500 species. The major center of diversification for Mimosa is Central Brazil, where many species are found in Caatinga and Cerrado vegetation.1,2 The Cerrado and Caatinga not only have one of the world’s highest levels of biodiversity but also has an enormous cultural diversity and an as yet under-used repertoire of plants with potential economic value.3
Mimosa caesalpiniifolia Benth (Fabaceae: Mimosoideae) is a medicinal plant popularly known as “sabiá” or “sansão-do-campo” is a rugged, fast-growing Brazilian Northeast native perennial tree and markedly contributes to production of pollen and honey, being considered an important honey plant in this region.4–6 In the Northeast region of Brazil, the leaves and/or the bark are prepared in form of infusion, decoction and “garrafadas” against inflammatory diseases, hypertension and infections,5,7,8 and the hydroalcoholic extract of Mimosa caesalpiniifolia leaves exhibited antimicrobial, antioxidant,9 cytotoxic activity against the human breast cancer cell line MCF-710 and the dose of 62.5 and 125mg/kg, reduced oxidative DNA damage was as show by single cell gel (comet) of blood cells of animals exposed to cadmium.11 Besides, their dried or green leaves are often used as fodder for sheep, goats and cattle, since they have high protein and minerals content.3
In addition, several other Mimosa species have been also used to treat other diseases.12 Mimosa pudica is used in the treatment of a variety of medical problems including anthelmintic action,13 anti- inflammatory properties14 and the root extract contains high levels of antioxidants. Mimosa tenuiflora is a popular remedy utilized in Mexico and Brazil, the bark of this plant once dried, powdered, and directly applied to the lesion is an effective remedy for treating skin burns and wounds and preventing inflammation15,16, Mimosa hostilis is used against cough and for wound healing17, Mimosa pigra is used to fight colds and flu, Mimosa humilis against rheumatism18,19 and Mimosa albida is employed for stomach problems and insomnia.
Phytochemical analysis in some Mimosa species showed the presence of flavonoids, saponins, alkaloids, terpenoids, non-protein amino acids (mimosa), tannins and fatty acids.20 Silva et al.,21 identified two important flavonoids from the leaves of M. caesalpiniifolia, catechin, 2,3 dihydroquercetagetine and procyanidin B2 [(epi)catechin- (epi) catechin] and the authors Silva et al.,22 identified 28 compounds, five of which are rare: 6-(β-boivinopyranosyl)apigenin, 8-(β-oliopyranosyl)-apigenin, (E)-6-(2-carboxyethenyl)apigenin, (E)-8-(2-carboxyethenyl)apigenin, and 7,5″-anhydro-6-(α-2,6- dideoxy-5-hydroxyarabinohexopyranosyl)apigenin in the leaves of M. caesalpiniifolia.
Due to the use of M. caesalpiniifolia leaves in folk medicine to fight inflammatory diseases, without scientific evidence of this potential therapeutic application, the objective of the study was to carry out a phytochemical profile and evaluate the properties of the hydroalcoholic extract of M. caesalpiniifolia (EEM) leaves responsible for its anti-inflammatory and antioxidant effects, which help EEM in the ethnopharmacological use in popular medicine.
Drugs and dosage
EEM was emulsified with saline solution (0,9% NaCl), before use. The animals were orally treated with EEM, at the doses of 62.5, 125 and 250mg/kg, p.o. Controls received vehicle and were administered by the same route as the treated groups. The following substances were used: ethanol (CPQ-Brazil), dexamethasone and xylene was purchased from Sigma-Aldrich Chemicals Company (St Louis, MO, USA). All chemicals used were of the analytical grade available and solubilized in saline solution.
Plant material and extraction
The leaves of M. caesalpiniifolia Benth, Fabaceae were collected in February, 2012 at the Fazenda do Rosa, Alfenas-MG, Brazil (21°24’44,1” S, 45°55’19,9” W).The plant was authenticated by Dr. Marcelo Polo and Dr. Geraldo Alves da Silva. Voucher specimens were prepared, identified, and deposited at the Herbarium of the UNIFAL - Universidade Federal de Alfenas (Alfenas - MG, Brazil) under code number 695. Leaves were completely dried at 40-45°C and pulverized in a knife grinder to fine powder (250µm - particle diameter;. The hydroalcoholic extract (EEM) was prepared using 70% ethanol by percolation method.23,24 The powdered leaves of M. caesalpiniifolia (260.0g) were percolated with 70% (v/v) ethanol at a rate of 1- mL/min until the extraction was exhausted. The extracts were combined, filtered and evaporated to dryness using a rotator evaporator under controlled temperature and reduced pressure (yield 24.15%).
Microencapsulation by spray drying of EEM
The EHM was dried in a Mini-spray dryer, Buchi® B191. The selection of operating conditions employed in the drying process was based on previous studies developed by prof. Dr. Geraldo Alves da Silva from the Federal University of Alfenas (UNIFAL-MG) and in the studies by Cortés-Rojas et al.25 To evaluate the drying parameters, dry extracts were obtained with different drying temperatures ranging from 120 ºC, 140 ° C and 160 ºC and in the concentration of 20% of adjuvant - Aerosil®, keeping the feed flow speed and the pressure Table 1.
Inlet temperature of drying air (Tge) =120 ºC, 140 ºC and 160 ºC |
Air pressure atomatization (Patm) = 1.5 bar |
Air flow automatization (Watm) = 10lpm |
Extract feed flow (Wsusp) = 1g/min |
Drying air flow (Wg) = 60m3/h |
Table 1 Operating parameters of spray drying
Heating the drying air is the first step at the beginning of the drying operation. When the desired temperature was reached, the water was started at the pre-chosen flow rate. The drying air outlet temperature was monitored for approximately 15 minutes, until the condition remained stable. When this condition is reached, feeding of the drying composition begins, with the outlet temperature monitored every 5minutes. During the performance of the drying tests, the ambient temperature and the relative humidity of the air (%) were monitored.
Chemical fingerprint of EEM
The mass spectrometry experiments were performed on LCQ Fleet equipment (Thermo Scientifics®) equipped with the dispersal of the directly introduced sample via flow injection analysis (FIA). The studied matrix was analyzed by electrospray ionization (ESI), and multiple stages of fragmentation (MS2, MS3, MSn) were performed at an ion trap (IT) interface. The positive mode was selected for the generation and analysis of the mass spectra for the first order (MS) and for the remaining multi-stage experiments under the following conditions: capillary voltage, 25V; voltage spray, 5kV; capillary temperature, 275 °C. A carrier gas (N2) with a flow of 8 arbitrary units (A.U.) was used, and the collision gas was helium (He). The track acquisition was 100-2000m/z. Xcalibur version 2.2 software (Thermo Finigans) was used to acquire and process the data. For the FIA-ESI-IT-MSn assay, 10 mg of EEM was dissolved in 1mL of MeOH:H2O (1:1, v/v) after using an ultrasonic bath for 5 min. The samples were then filtered through a 0.22mm PTFE filter, and aliquots of 20mL were directly injected into the FIA-ESI-IT-MSn system.
Antioxidant activity of EEM
The antioxidant activity of EEM was determined by using both the 1,1-diphenyl-2-picrylhydrazyl (DPPH) radical scavenging and the oxygen radical absorbance capacity (ORAC) in vitro assays, as previously described.8,26 Tested compounds and, standards were diluted in absolute ethanol at different concentrations from stock solutions of 1 mg/mL in DMSO. Aliquots (100µL) of these diluted solutions were placed in 96-well plates in triplicate for each concentration tested, and then read at 517nm, using a UV/Visible spectrometer BioTek® (ELISA). Ethanol was used as a blanck, and 10, 25, 50 and 75µM of Trolox (hydrophilic α-chlorogenic acid was used as quality control. The DPPH-scavenging activity of the tested compounds was compared to that of the Trolox calibration curve. The results were expressed as Trolox equivalent (micromoles of Trolox equivalents per gram of dry matter).
Oxygen radical absorbance capacity (ORAC) assays were carried out according to the method previously described with some modifications.8,27 Samples were analyzed in triplicate and diluted to different concentrations (25, 12.5, 6.25 and 3.12µg/mL) using a stock solution of 1mg/mL in DMSO, using a UV/Visible spectrometer BioTek® (ELISA). The final ORAC values were calculated using a regression equation between the Trolox concentration and the net area under the fluorescein decay curve and expressed as micromoles of Trolox equivalents per gram of dry matter. The AUC was calculated using Magellan™ data analysis software.
Animals
Male Swiss mice (40-50g) were used for the anti-inflammatory experiment, respectively. The animals were obtained from the Central Animal House (UNESP) in Botucatu, SP. and housed in the Physiology Department under controlled temperature (23±2ºC) and a 12 h light/dark cycle. They were provided a certified Labina (Purina, Brazil) diet and tap water ad libitum. Before each experiment, the animals were deprived of food for either 6 or 12h as described in each experimental model. Standard drugs and the EEM were administered orally using a saline solution (0,9% NaCl, 10mL/kg) as the vehicle. The UNESP Institutional Animal Care and Use Committee approved all of the employed protocols (Protocol 656/657-CEEA) (Collaboration - Partherhip: Prof. Dr. Clélia Akiko Hiruma-Lima, Departamento de Fisiologia, Instituto de Biociências, UNESP).
Evaluation of anti-inflammatory activity
Xylene-induced ear edema: This experiment was performed as described by Swingle et al. with modifications. For induction of ear edema, 40µl xylene were applied topically to the right ear of the mice (20µl on the anterior surface of the ear and 20µl the posterior surface). The left ear was used as control. The animals (n=8-10) were treated 2hours before induction of edema with dexamethasone (5mg/kg, i.p), one hour before induction were given orally the HECS (62.5, 125 or 500mg body weight) and the negative control (vehicle). After one hour of induction of edema, the mice were killed and a circular section (diameter 7mm) was taken from its ears left and right with the help of a puncher (punch) and immediately weighed. Edema was expressed by the difference in mass (in milligrams) between the right and left ear.
The EEM was prepared with the addition of the technological adjuvant in a proportion of 80:20 (w/w) of Aerosil® 200 and the dry residue was given with 8% dry extract. In the literature there are several works using Aerosil® 200 to dry plant extracts. This adjuvant is widely used used in topical and oral pharmaceutical products due to its lack of toxicity.
The preliminary phytochemical screening of EEM showed the presence of flavonoids, terpenes, gallic acid and catechins9,10 and in the present work, the analysis of EEM by ESI-IT-MS with direct insertion (FIA) the full scan spectrum, positive way confirmed the presence of flavonoids glycosides (m/z 563, 579, 621) and derivatives of catechin (m/z 595, 611, 741 and 757) (Figure 1). The fragmentation of the second order (MS2) ion at m/z 741shows the loss of three sugar units ([M-deoxihexose 2 - hexose + H]+), resulting in a glycone ion m/z 287 ([A+H]+). The ion m/z 757 shows loss of three sugar units ([M-deoxihexose 2 - hexose + H]+), resulting in a aglycone m/z 303. According to the literature, ions of the aglycone m/z 287 and 303 correspond to kaempferol and quercetin respectively.
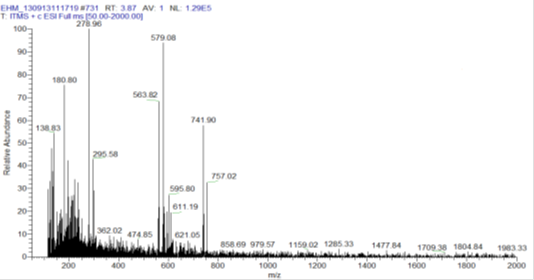
Figure 1 First-order mass spectrum of hydroalcoholic extract of M. caesalpiniifolia (EEM) leaves at the positive mode. Range of ions with m/z of 100-2000 Da.
For the ion m/z 595 the loss of two units of sugar ([M-deoxihexose - hexose + H]+), resulting in its aglycone ion m/z 287. The ion m/z 611 the loss of two units of sugar ([M-deoxihexose - hexose + H]+), resulting in its aglycone m/z 303. The ions m/z 563 and 579 were identified as catechin derivatives, which are substances that exhibit potent antioxidant activity with the elimination of free radicals and are anticarcinogenic anti-inflammatory agents, antidiarrheal, and antimutagenic.12,28,29 The other ions detected were also subjected to experiments of MS/MS. In some cases, the spectra showed fragment ions m/z 303 or 287, referring to the glycosylated flavonoid and aglycones.
Antioxidant potential of EEM leaf was ass assessed by both DPPH scavenging and ORAC assays on EEM leaf hydroalcoholic extract. Antioxidant properties of this extract are same as those obtained for Psidium guajava leaf hydroalcoholic extract (Table 2), containing anthocyanins, flavonoids, proanthocyanidins, sesquiterpenoids and triterpenoids, which are accepted as the strongest antioxidative compounds.30,31 The antioxidant activity of a given compound or plant extract is also often associated with its radical-scavenging activity. These results provide evidence for antioxidant and anti-inflammatory, antidiarrheal properties of EEM leaf hydroalcoholic extract.
|
Extract or compound |
DPPHb (µmol TE/g) |
ORACb ((µmol TE/g) |
|
EEM hydroalcoholic extract |
1765±6 |
3084±28 |
|
Psidium guajava hydroalcoholic extracta |
392±14 |
3335±56 |
|
Quercetin |
7848±5 (2.87±0.013µmol TE/µmol) |
29,108±502 (8.22±0.22µmol TE/µmol) |
|
Chlorogenic acida |
3081±18 (1.81 ±0.04µmol TE/µmol) |
15,523±195 (6.05±0.03µmol TE/µmol) |
Table 2 Antioxidant activities (DPPH and ORAC assays) for EEM hydroalcoholic extract and pure compounds.
aReferences (Frankel et al., 1996; Fernandes, 2013 Flores et al., 2015).
bValues are mean ( ± SD) of their independent experiments.
The animals pretreated with EEM showed significant reduction of edema only at the 125 and 250mg/kg doses, inhibiting 52% and 64% respectively, when compared to the group treated with the vehicle (saline) as shown in Figure 2.
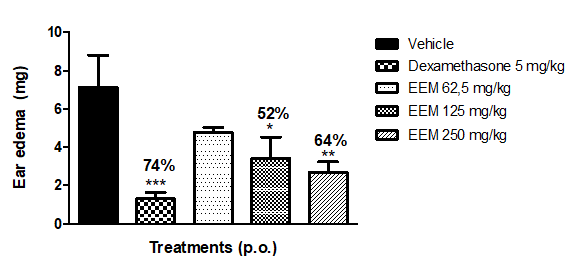
Figure 2 Effect of M. caesalpiniifolia hydroalcoholic extract (EEM) (62.5; 125 and 250 mg/kg, p.o.) in ear edema model induced by xylene. Results are expressed as mean ± S.E.M. and statistical significance was determined by ANOVA followed by Dunnett's test; *p<0.05, **p<0.01, ***p<0.001 compared to the control group (vehicle). Percentage corresponds to the reduction of the mean difference in weight (mg) of the ear in the control group (vehicle).
Encapsulating agents can be of natural, semi-synthetic or synthetic origin, including polymeric, hydrophilic, hydrophobic materials or an association of both. Encapsulating agents have the function of providing protection during prolonged storage, preventing chemical and sensory changes from occurring in the encapsulated material.32
In studies carried out by Vasconcelos,33 2005 with Aerosil®, they showed that the dry extracts showed good physical stability, maintaining the aspect of fine and loose powder, in addition to providing greater yield to the process. Therefore, Aerosil was the adjuvant of choice.
The present study aimed to evaluate the phytochemical profile and anti-inflammatory and antioxidant of EEM in rodents. Furthermore, no reports were found in the literature regarding these biological actions of M. caesalpiniifolia and although some pharmacological actions of the Mimosa species have been reported, no specific anti-inflammatory of M. caesalpiniifolia have been reported in the literature. This study provides a pharmacological basis for M. caesalpiniifolia use in folk medicine and shows that this plant has potential for the development of phytomedicines.
The phytochemical screening revealed the presence of secondary metabolites such as flavonoids. The flavonoids found in this species are predominantly flavonol glycosides with quercetin and kaempferol skeleton previously identified in species of Mimosa. As in M. caesalpiniifolia two flavones [C-glycoside-isovitexin-2”-O-ramnopiranoside and vitexin-2”-O-ramnopiranoside] were detected in M. xanthocentra.34
To evaluate the anti-inflammatory effect of EEM, was performed the model of ear edema induced by xylene. This model is an advantageous method in testing natural products and in the screening of anti-inflammatory agents, particularly those which inhibit phospholipase A2.35 Animals treated orally with EEM showed significant reduction in ear edema at doses of 125 and 250mg/kg in a dose dependent manner. This potent anti-inflammatory effect exerted by EEM may be due to the presence of glycosylated flavonoid and derivatives of catechin, known to be capable of suppressing the expression of mRNA of iNOS and COX-2 and thus decrease PG levels and NO.36–38 Our data are according to published works on different species from the Mimosa genus that described the presence of flavonoids which are responsible for different biological activity, including anti-inflammatory and antioxidant activities.8,39
Phytochemical analysis of EEM showed the presence of flavonoids, saponins, alkaloids, terpenoids and tannin, which have been reported to be present in other plants with anti-inflammatory properties.22,40 It is possible that the bioactive compounds present in the hydroalcoholic extract of the leaves of M. caesalpiniifolia are responsible for the anti-inflammatory and antioxidant effects described for the extract. Particularly flavonoids, which have the ability to capture free radicals, can overcome the effects of known antioxidants, such as, for example, vitamins A and E.
On this occasion, these results led us to investigate the possible gastroprotective effect in models of acute gastric ulcer. Thus, the gastroprotective effect was evaluated through the absolute ethanol and indomethacin-induced gastric lesions. EEM showed no protective activity on either model. The presence of the glycosylated flavones and catechin derivate may also explain this lack of gastroprotective activity since the inhibition of both COX-2 and NO is responsible for the development of gastric lesions.41
The results of this study contributed not only to the initial development of a pharmaceutical pre-formulation with natural products, but also in the search for new anti-inflammatory drugs.The results of this study contributed not only to the initial development of a pharmaceutical pre-formulation with natural products, but also in the search for new anti-inflammatory drugs. Whereas the substances identified may be responsible for the protective effect against the risks of many pathological processes, the results described in this study demonstrate that the species M. caesalpiniifolia presents itself as a promising source of substances with activities antioxidant and anti-inflammatory. Further studies are needed to define the mechanisms of action of the anti-inflammatory activity of M. caesalpiniifolia leaves. However, the results corroborate the popular use of this plant.
All authors declare there are no conflicts of interest.
Financial support from FAPESP (Proc. 2012/18760-9) and (Proc.2015/21479-8). CNPq (Proc. 151634/2019-00).

© . This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.