International Journal of
eISSN: 2381-1803


Research Article Volume 11 Issue 3
1Animal Health Research Institute, Assiut Lab, Egypt
2Plant Protection Research Institute Dokki Cairo, Egypt
Correspondence: Abdul-Hafeez MM, Plant Protection Research Institute Dokki Cairo, Egypt
Received: May 04, 2018 | Published: June 15, 2018
Citation: Hamouda SM, Abd ERMF, Abdul-Hafeez MM, et al. Egyptian fennel honey and/or propolis against MRSA harboring both mecA & icaA genes. Int J Complement Alt Med. 2018;11(3):180-185. DOI: 10.15406/ijcam.2018.11.00392
Food methicillin resistant Staphylococcus aureus (MRSA)is public health zoonotic multidrug resistant pathogen and when encoded biofilm production gene, it will be more virulent stubborn strain and difficult to be eradicated. So, searching for a potent natural antimicrobial agent that fights biofilm production is of great concern. Since honey or propolis has antimicrobial and antibiofilm activity against MRSA, the study aimed to examine these natural apiproducts against food biofilm producing multi–antimicrobial MRSA. Nineteen MRSA strains harboring both mecA & icaA genes (12 from food of bovine sources and 7 from food workers) were chosen to test in vitro the antimicrobial potency of Egyptian fennel honey and/or propolis against them. Multi antimicrobial resistance (MAR) indices and biofilm production were determined for these tested strains to conclude that having both virulence characters pheno–and genotypically. MAR index of tested strains was 0.48±0.12 where food strains was 0.46±0.12 and food worker strains was 0.51±0.13. All 19 MRSA were biofilm producers on Congo red agar. Against all 19 strains, both tested apiproducts (Egyptian fennel honey and EEP [Ethanolic Extract Propolis]) resulted high antimicrobial activity where honey–alone–was more potent (8.21±3.35%) with very highly significant difference (P<0.001) than EEP (14.3±3.48%) and showed strong synergistic effect when be added to EEP (7.84±2.52%). Against food MRSA honey MIC showed (7.91±3.5%) and honey with propolis was (7.33±2.42%), but against food worker's MRSA, honey MIC showed (8.71±3.3%) and honey with propolis was (8.71±2.63%). It was concluded that fennel honey and/or propolis have potent antimicrobial activity against foodborne pathogens especially biofilm producing MRSA and recommended these nutritive apiproducts as food additives and preservatives.
Keywords: Fennel honey, food MRSA, resistance, biofilm
Methicillin resistance S. aureus (MRSA) isolated from various foods of bovine origin is of great concern about possible dissemination throughout the food production chain.1 MRSA resists β–lactams antibiotics (penicillins, cephalosporins, monobactams, and carbapenems groups)2 which are primarily conferred by the acquisition of mecA gene encoding penicillin binding protein (PBP 2a).3 This protein is an important factor in biofilm accumulation,4 then MRSA adhere to biotic or abiotic surfaces5 and be protected against hostile environments.6 MRSA antimicrobial resistance might be increased on harbouring any of biofilm producing gene (ica operon).7,8 MRSA such recalcitrant biofilm producers are 1000–fold more resistant to antibiotics and immune defense cellular elements.9 Moreover, biofilms act as reservoirs of pathogenic microorganisms resulted in biomass formation difficult to be eradicated.10 So, searching for antimicrobial agent that fights biofilm production is of great concern. Against MRSA, not only honey11–21 or propolis22–27 has antimicrobial activities, but also they have tremendous antibiofilm activities28–31 which are widely studied and documented. Since Egyptian fennel honey has potent antimicrobial activity against S. aureus,32–34 the study aimed to study the antimicrobial activity of Egyptian fennel honey and/or propolis against these stubborn biofilm producing MRSA recovered from food and food workers.
Bacterial strains from a previous work,35 nineteen MRSA strains harboring 16srRNA as well as virulence genes (mecA & icaA) were chosen and included in the present work. Twelve MRSA strains originated from food of bovine origin (9 dairy and 3 meat sources) and the other seven strains were recovered from the same food workers (3 throat and 4 fingernails sources). All MRSA strains were subculture onto Congo red agar for biofilm detection phenotypically.
Tested apiproducts: fresh Egyptian unifloral fennel honey–kept in a dark bottle away from any source of heat–and ethanolic extract of Chinese propolis powder (EEP) were used to be tested again the chosen MRSA strains. EEP extract standard solution was prepared as 40g of propolis powder was extracted as described,36 then the extracted amount was dissolved in 100ml of deionised water to be considered a standard EEP solution (100%) and be sterilized just after preparation the wanted dilution to be added to the melted agar. Multi antimicrobial resistance (MAR) index: to calculate MAR index of MRSA strains, antibiotic sensitivity testing as McFerland standards 0.5%37 was prepared for disc diffusion sensitivity testing according to the Kirby–Bauer method38 using discs: erythromycine (15µg), ciprofloxacine (5µg), sterptomycine (10µg), pencillin G (10 IU), amoxacillin (35µg), chloramphenicol (30µg), gentamycine (10µg), sulphamethaxazole (20µg), Oxacillin (1µg), novobiocine (30 ), doxycycline(30µg), cefotaxime(30µg), amekain (30µg) and polymyxin B (300 IU)–Bioanalyse, Turkey. Resistance was judged by the inhibition zone diameter ø to determine the MAR index that was idefined as a/b, where (a) represents the number of antibiotics to which the isolated strain was resistant and (b) represents the number of all tested antibiotics.39, 40
Honey and propolis minimum inhibitory concentration (MIC) determination: honey or EEP was dissolved in sterile deionized water to prepare a stock solution of 20% v⁄v honey immediately before each use. Further dilutions were prepared by adding honey and sterile deionized water to sterile 10–ml volumes of molten double–strength nutrient agar at 50°C and pouring immediately to produce a range of plates containing honey at 1% (v⁄v) intervals between 0 and 20% v⁄v.41 For testing the synergistic effect of both tested apiproducts, honey and EEP were added as described above but with half amount for each to obtain the final concentration of mixed products. Plates were dried at 37°C for 15 min before use. McFerland standards 0.5% from overnight broth culture of MRSA was used for MIC determination as each MRSA standard dose was inoculated onto every prepared concentration of api– product plate. Plates were incubated at 37°C for 24h before visual assessment.41
MRSA biofilm–associated infections are difficult to be eradicated because the biofilm is strongly resistant to wide variety of antibiotics and the host immune response.3 The vast majority of food–borne outbreaks caused by antimicrobial–resistant pathogens are the result of the consumption of contaminated foods of either animal–origin or multi–ingredient foods.42 In the present study, MAR index among food strains, MRSA from meat showed the highest value as 0.61±0.1 (Figure 1), while among food worker strains, fingernail strains showed 0.57±0.14. Figure 2 and the overall MAR index of tested strains was 0.48±0.12 (Figure 3). The tested MRSA strains had very high MAR index value since MAR index value just≥0.2, is considered high40 and might be originated from environments with misuse of antibiotics where resistance developed and spread,43 rather than harboring both mecA & icaA genes (Figure 4). All tested MRSA strains showed positive congo red testing concluding that these tested strains were biofilm producing MRSA pheno as well as genotypically. So, these nineteen MRSA strains represent different hazard sources threating public healthcare classified as multidrug resistant, biofilm producer foodborne pathogens.
In the present study, the in vitro apitherapy testing against these stubborn strains resulted that both tested apiproducts showed good safe promising anti MRSA activity. Fennel honey showed MICs values much less than those of propolis all over the present study against food MRSA strains as (7±3.6 & 10.66±1.15%) for dairy and meat food, while against food worker’s MRSA as (9.33±3.05 & 8.25±3.8%) for throat and fingernail contents respectively (Figures 5 & 6). EEP MICs against dairy and meat food MRSA isolated were higher than honey MICs and lower potency as (13.8±4.05 & 15.33±2.31%), while against food worker’s it was (12 & 16.5±3.41%) from throat and finger nail contents respectively (Figure 5) (Figure 6). Against all tested MRSA strains, fennel honey also revealed MICs values (7.91±3.5 & 8.71±3.3%) much lower than that of EEP (14.17±3.66 & 14.57±3.41%) against food and food worker’s respectively (Figure 7). S. aureus is highly sensitive either to honey44 or EEP45 than other Gram positive and Gram–negative bacteria. In vitro antimicrobial honey activity against MRSA11–21 with wide varied potencies of MIC ranging from 3.1 up to 25% or inhibition zone20,46,47 of 8–28mm. These differences are depending on botanical, geographical and seasonal conditions48 leading to differences in antimicrobial potency more than 100–fold in–between different honeys.49 Also, propolis MIC has more widely range from 1.0 up to 10027,45,50–53 and may be of very weak potency reaching≥50054 mg/L depending on very wide range of different propolis constituents from different geographic regions.24,27,52 Honey possesses antibacterial activities depending on physicochemical properties such as osmotic pressure, low pH of 3 to 4.5 and non–peroxide factors (phytochemicals) as polyphenols,55 as well as peroxide effects due to H2O2 level in honey which is a strong predictor of its antibacterial activity.56 H2O2 is involved in oxidative damage causing bacterial growth inhibition by DNA degradation and modulated by other honey components.56 Honey seems to induce DNA damage when H2O2 and phenolic compounds act in synergistic mechanism by a putative pro–oxidant effect57 but H2O2 could be reduced when honey is processed.58 Honey is rich in phenolic acids as caffiec and flavonoids mainly chrysin which exhibit a wide range of biological effects59 occurring naturally in honey and propolis.60 Honey interrupt and inhibit MRSA cell division by the action of sugars and methylglyoxal as additional in vitro antimicrobial activity,61 moreover, cell membrane could be destructed by the action of Sidre honey, resulted in releasing of bacterial cellular proteins.62 Micro components honey glycoprotein fractions exhibited strong growth inhibitory and bactericidal properties possessed two distinct functionalities: specific binding and agglutination of bacterial cells, and non–specific membrane permeabilization bacterial cells.63 The antibacterial activity of honey is highly complex due to the involvement of multiple compounds and due to the large variation in the concentrations of these compounds among honeys.64 Other micro components: methylglyoxal (MGO) and antimicrobial peptide bee defensin–1.64 The presence of MGO can modify some honey proteinaceous compounds and therefore can affect the glucosidase activity.65
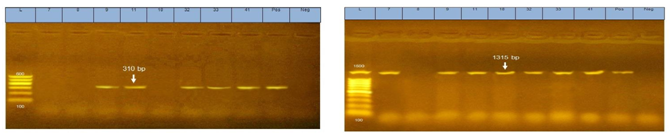
Figure 4Agarose gel electrophoresis of PCR for Mec A gene at 310bp and icaAgene at 1315bp encoded in MRSA recovered from food and food workers.
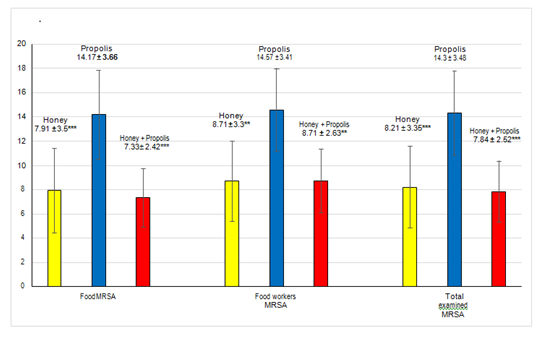
Figure 7 MIC of apiproducts against total examined MRSA.
***Very highly significance difference P<0.001; **Highly significance difference P<0.0
Against S. aureus, propolis has different antibacterial mechanisms, including inhibition of cell division, collapsing microbial cytop lasm cell membranes and cell walls, inhibition of bacterial motility, enzyme inactivation, bacteriolysis, and protein synthesis inhibit ion.66 Bioactivities of EEP where major constituents exhibited polyphenols, aromatic acids, terpenes and flavonoids27 are not directly related to its concentration, but a synergistic activity53 and interaction between these various active ingredients is believed to be a main factor in achieving the complex antimicrobial activity of propolis.27 Antibacterial activities are attributed to the flavonoid formononetin,67 while polyphenols interacts with many microbial proteins by forming hydrogen and ionic bonds, thus altering their three–dimensional (3D) structure of a protein and as a consequence their functionality.27 Micro components (diterpenes) isolated from propolis possess antibacterial activities.68 The potent bacteriostatic and bactericidal effects of propolis can be associated with their combined action, manifested by an inhibition of protein synthesis and bacterial growth by preventing cell division.69 Antimicrobial synergistic activity during the present work was noticed when use both added apiproducts either against dairy and meat food MRSA as (7.11±2.8 & 8%), while against food worker’s MRSA it was as (10 & 7.75±3.3%) for throat and fingernail contents respectively–Figure 5 & 6). Against all tested MRSA strains, fennel honey and EEP showed synergistic activity as MICs (7.33±2.42 & 8.71±2.63%) for food and food worker’s respectively. Against all tested MRSA strains, fennel and EEP achieved MIC of 7.84±2.52% (Figure 7) resulting that fennel honey is potent anti MRSA than EEP, while both have activity but showing strong synergistic activity when be added together. Honey antimicrobial activity23, 34 or healing promotion70 has synergized with EEP or antibiotics {vancomycin,62 gentamycin,71 rifampicin,31 and oxacillin61} against MRSA. Not only using antibiotics in combination with honey have synergistic antibacterial action, but also it ought to reduce risks of further antibiotic resistance emerging since application of honey in combination with oxacillin would restore MRSA sensitivity61 through regulation of mecA by blocking mecR1–mediating signaling pathway.72 Honey also has synergistic antimicrobial action with natural materials as chitosan73 and ginger powder extract.74 Otherwise, EEP showed synergistic antimicrobial effect with antibiotics {oxacillin,27 clarithromycin,75 cefazolin,76 ciprofloxacin50 cefixime77 and many different antibiotics27,53,66,69} or essential oils of ginger,78 garlic,79 cinnamon,80 clove,78 or chitosan.81
The study concluded that Egyptian fennel honey and EEP have potent antimicrobial activity against biofilm producing MRSA, while both apiproducts revealed more potency and synergy will be achieved against these foodborne pathogens. It is recommended to use the nutritive materials (fennel honey & EEP) as food preservatives against foodborne pathogens especially biofilm producing MRSA.
None.
Author declares that there is no conflict of interest.

©2018 Hamouda, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.