International Journal of
eISSN: 2381-1803


Research Article Volume 8 Issue 4
1Pharmacy Department, Orient University, Cuba
2Universidad del Valle. Toluca, México
3Toxicology and Biomedicine Center, Medical Sciences University of Santiago de Cuba, Cuba
4Instituto de Farmacia y Alimentos, Universidad de la Habana, Cuba
Correspondence: Jesús Rafael Rodríguez Amado, Post Doc student, Amapá Federal University, Macapá, Amapá, Brazil
Received: October 29, 2016 | Published: August 24, 2017
Citation: Amado JRR, Prada AL, Arranz JCE, Keita H, Zapata EP et al. (2017) Development and acute Oral Toxicity Evaluation of a dry Extract from Tamarindus Indica L. Int J Complement Alt Med 8(4): 00267 DOI: 10.15406/ijcam.2017.08.00267
The necessity for obtaining dry extracts from natural products is evident. For this, most useful methods are spray-drying and freeze-drying. The last is long and expensive. The former is fast but also expensive. The aim of this work was to obtain a dry extract from Tamarindus indica L fluid extracts, by a simple technique at laboratory scale, which can be used properly in pharmaceutical compounding and to evaluate the acute oral toxicity of it. Lactose monohydrate and colloidal silicon dioxide were used as adjuvants to improve the drying process. The best proportion of the adjuvant was achieved using a Central Composite 22 full factorial design. The acute oral toxicity was evaluated in Wistar rats, by using the OECD 425 methodology. The optimized proportion of the adjuvant was 1.14 g of colloidal silicon dioxide and 25.0 g of lactose monohydrate. The colloidal silicon dioxide plays an essential role on the physicomechanical properties of the dry extract. The extract was framed as a non-toxic substance (Not classified) according to the scale of toxicity of OECD 425. Thus, the present study allowed obtaining a dried extract with excellent physicomechanical properties, by using a simple’s method.
Keywords: natural products, physic-mechanical properties, lactose monohydrate, colloidal silicon dioxide, central composite design
DB: bulk density; DT: tapped density; CI: compressibility index; HI: hausner index; FR: flow rate; AR: repose angle; HR: Residual Humidity; PS: particle sizelm; lactose monohydrate; CSD: colloidal silicon dioxide; GP: goodness of prediction, goodness of fittde; TDE: tamarind dry extract
Nowadays, several medicinal plants are used to produce pharmaceuticals. Nonetheless, development of phytopharmaceutical has intrinsic technological challenges.1 Concerning to liquid and dry plant extracts, the latter possess many advantages over the former. The chemical and microbiological stability are greater in dry extracts. However, the dry extracts usually show poor physicomechanical properties and very poor compressibility. The dried extracts are easily affected by moisture and usually are not able to be used in the manufacture of tablets by direct compression.2 The drying of vegetal extracts can be successfully improved if some excipients are used as drying carriers.1 The most used excipients in this context are colloidal silicon dioxide, malt dextrin, and lactose and corn starch.3 If the drying adjuvants are well selected, the flow properties and compressibility of the dry extracts can be improved, allowing that can be utilized, in the formulation of solid dosage forms.4 Currently, almost all published articles describe the method to obtain natural dry extracts by using spray drying or freeze-drying. The last is long and expensive. Just a few material justify the preparation by this way. The former is fast but also expensive. The authors mainly describe the obtaining methods at the laboratory level. In this sense, to find a simple method to obtain dry vegetal extracts ready to use in pharmaceutical compounding, by using a simple technology; that can be found in everywhere; is a challenge for the technologist.
Tamarindus indica L. (Tamarind) belongs to the family Fabaceae, is a native evergreen tree from tropical areas of Africa and Southeast Asia. T. indica is widespread in the Caribbean, where is widely used for the treatment of liver disorders.5 The ethno botanical use of tamarind in Cuba and Brazil is widespread and this species represent one of the most common remedies for the treatment of hepatitis. Leaves from T. indica are rich in polyphones, flavanoids, ferulic and caffeic acid.6 Polyphenols and flavonoids have been associated with the antioxidant effects,7 hepatoprotective.7,8 and antimicrobial of this plant.9 Until this moment, there is not any attempt in literature to obtain a dry extract of this plant. Thus, is essential evaluated, if the new dried extract obtained is non-toxic. The aim of this work was to obtain and evaluate the oral acute toxicity of the tamarind dry extract (TDE), by using lactose monohydrate and colloidal silicon dioxide as drying adjuvants.
Plant material
The tamarind leaves were collected in El Caney, Santiago de Cuba, Cuba (020° 2' 38.9 " N - 075° 45' 25.8" W). Identification was performed by Dr. Jorge Sierra Calzado and a voucher of the specimen was deposited at the Herbarium of the Department of Biology, Faculty of Natural Sciences, University of Orient, Cuba, under the register number 052216.
Dry extract preparation
The fluid extract was obtained using ethanol 72 % (v/v).10 The extract was concentrated under vacuum (IKA HB 06.05, Switzerland) until to obtain a relation of 4g of dry drug per mL of the extract.11 The selected amount of lactose monohydrate (LM) (Contero Excipient, New Zealand) and colloidal silicon dioxide (CSD) (Aerosil® V-200, Degusa, Belgium), were weighed and placed in a mortar. They were carefully blended and sifted through a sieve of 250 µm. Then, an amount of concentrated extract equivalent to 30 g of total solids was added to the powders mixture. These ingredients were mixed and granulated by using a KG-5 Bench Top High Shear Granulator/Mixer (Key International Inc., NJ. USA) by using an opening of 0.84 mm. Drying was carried out in a vacuum oven (Nova Etica, Brasil) at 40 ± 2ºC for three hours. A -1080 mmHg vacuum was used. The dried extract (TDE) was cooled to room temperature for 24 h. Subsequently, the whole mass was milled in a bench mill (FitMill, USA) until it passed through a sieve with a 250 µm. The performance equipment´s parameters were held constants.
Experimental design
A 22 full factorial central composite design, with three central points (Table 1) was used to assess the effects of factors Lactose monohydrate (20g-30g) and Colloidal silicon dioxide (0.3g-1g) over the physicomechanical properties of TDE.12 The responses used were bulk density (Db), tapped density (Dt), compressibility index (CI), Hausner index (HI) flow rate (Vf), repose angle (Ar), residual humidity (Hr) and particle size (PS).
|
Nº |
Point Type |
Lactose |
Colloidal Silicon |
|
1 |
Factorial |
20,00 |
0.30 |
|
2 |
Factorial |
30,00 |
0.30 |
|
3 |
Factorial |
20,00 |
1.00 |
|
4 |
Factorial |
30,00 |
1.00 |
|
5 |
Axial |
17.93 |
0.65 |
|
6 |
Axial |
32,07 |
0.65 |
|
7 |
Axial |
25,00 |
0.16 |
|
8 |
Axial |
25,00 |
1.14 |
|
9 |
Center |
25,00 |
0.65 |
|
10 |
Center |
25,00 |
0.65 |
|
11 |
Center |
25,00 |
0.65 |
Table 1 Experimental design matrix of the design showing the adjutants proportions used
Physicomechanical properties
Densities, compressibility and hausner indices: Bulk density and tapped density were assayed by a classical method proposed in the USP.13 In the same way, compressibility index (CI) and Hausner index (HI) were calculated according to USP.13 Measurements were performed in quintuplicate.
Flow rate: Flow rate (Vf) is a measure of the flow ability of powder and granulates to flow through the hoppers. It was measured by using a glass funnel with an orifice diameter of 8 mm. The angle of the walls of the funnel was 45°. The flow time through the funnel of a mass of 10 g of TDE was measured. Measurements were performed in quintuplicate. Vf was calculated using the following expression:
Vf = m / 0.785 x d2 x t
Where: m is the mass of TDE (grams); t is the time taken for the mass of TDE to flow through the funnel (seconds); d is the diameter of the orifice of the funnel (cm) and 0.785 is a numerical constant.14
Repose angle: The repose angle (Ar) was evaluated by dropping TDE through a glass funnel with orifice internal diameter of 8 mm, over a horizontal surface from a height of 100 mm. The height (H) of the powder mass formed and radius (R) of the base of the formed cone were measured.13 Measurements were performed in quintuplicate. The Ar was calculated using the following expression:
Ar = tan-1 (H/R)
Residual humidity: The residual humidity (Hr) was determined by the gravimetric method.13 using a gravimetric balance (Sartorius SA 325, Germany) with a precision of 0,0001g. Measurements were performed in quintuplicate.
Particle size: Particle size (PS) was determined using a vibrating sieve (THYL, Russia) with different apertures (250, 210, 177, 149 and 125 microns). The cumulative frequency was calculated and measurements were performed in quintuplicate. The average of PS was estimated using the following expression.
PS = (ni.Xi) / N
Where: “ni” is the mass of particles in the ith sieve; Xi is the opening arithmetic mean of two consecutive sieves and N, is the total mass of TDE. It was verified in each analysis that the whole mass of TDE used to traverse the top sieve.15
Optical microscopy
The particle shape was assessed by optical microscopy. Microphotographs of the selected dry extract were taken using an optical microscope by using an Olympus microscope (CBA, Japan). Images were automatically captured and processed using a magnification of 10x. (Software EDn-2, v.2.0.4, China).
Total phenolic and flavonoids
The total amount of polyphenol present in the selected dry extract was evaluated by spectrophotometry at 700 nm, by using the Folin Ciocalteu method.16 The analytical procedure was performed using pyrogallic acid (Sigma Aldrich, USA) as standard. Flavanoids were determined by spectrophotometry at 430 nm,16 using quercetin as standard. A spectrophotometer (QUIMIS, AA6300. Shimadzu, Japan) was used. All measures were made in triplicate. The total phenolic content was expressed in percent of pyrogallic acid. The flavanoids content was expressed as percent of quercetin. Both methods were performed and validated for this study.
Evaluation of acute oral toxicity
Animals: Wistar male rats were used, aged 10-14 weeks and weighing 160-180 g. The animals were supplied by National Center for the Production of Laboratory Animals (CENPALAB), Havana. They were acclimated for 7 days under standard conditions of temperature (22 ± 3 °C), relative humidity (60 ± 10%) and light-dark cycle (12/12 h). They were fed ad libitum with rodent chow (CMO-1000, CENPALAB) and distilled water. The test was conducted in accordance with Good Laboratory Practice for not clinical Laboratory Studies.17 The considerations established by the Ethics Committee of the Center for Toxicology and Biomedicine (TOXIMED), Medical University of Santiago de Cuba, Cuba were taken into account.
Acute oral toxicity test: The method of Acute Toxicity Class (ATC).18 was used. One control group and one experimental group (n = 7) were used. The experimental group was given the limit dose of 5000 mg / kg of dried extract, suspended in an aqueous solution of carboxymethyl cellulose (0.5%). The control group only received the used vehicle and standard diet. It was observed for 14 days, the mobility of the animals and the possible occurrence of death. Changes in the skin and coat, as well as the color and appearance of the mucous membranes and eyes, were observed. The daily uptake of water and food was recorded. Animals were weighed the days 0, 7 and 14 in a technical balance (Sartorius, Germany). After this time, they were sacrificed by using an intramuscular dose of ketamine, and it proceeded to a macroscopic pathologic examination of all organs. The toxicity classification was performed according to the criteria (Table 2) established in the regulation OECD 425.18
|
Acute Toxicity Classe (ATC) |
DL50 Range (mg/kg) |
Classification |
|
ATC 5 |
DL50 > 2000 |
No classify as toxic |
|
ATC 4 |
300 <DL50 < 2000 |
Dangerous |
|
ATC 3 |
50 <DL50 < 300 |
Toxic |
|
ATC 2 |
5 <DL50 < 50 |
Very toxic |
|
ATC 1 |
DL50 < 5 |
Highly toxic |
Table 2 Classifications of the oral Acute Toxicity Class as OECD 425
Data analysis
For data analysis, Design Expert 6.0 software was employed. The polynomial analysis was performed using partial least squares (PSL). The significant coefficients of each model were determined using the cross-validation technique (CV). The goodness of fit (R2) and goodness of prediction (Q2) were the criteria for the selection of adjusted mathematical models for each response. It was considered differences no more than 0.3 between Q2 and R2 (12). Confidence interval of 95% (p <0.05) was used. All responses (Y) were fitted to a second-order polynomial of the type:
Y = β0+ β1X1 + β2X2 + β1,2 X1X2 + ε
Where Y is the response, β0 is the intercept, β1, β2, are the magnitude of the effect of the factors CSD and LM, and β1,2 the magnitude of the interaction between the factors. A t test or F test was made, as needed, for the mean comparison between two groups of data (the former) and for more than of two groups of data (The last) by using StatGraphics XVI. Centurion (StatEasy Co, USA).
Physicomechanical properties of the dry extract
Table 3 & table 4 shows physicomechanical properties of TDE obtained with the experiment design and the coefficients of each set values for each response, respectively. Table 3. Results matrix of the experimental design with the standard deviation (n = 5). Table 4 Quadratic model adjusted for each response in the tamarind dry extract.
|
Nº |
Db |
Dt |
Ar |
Vf |
Hr |
PS |
CI |
HI |
|
1 |
0.539 |
0.799 |
40.25 |
6.55 |
2.17 |
226.1 |
32.54 |
1.48 |
|
2 |
0.479 |
0.753 |
35.71 |
6.71 |
2.39 |
230.5 |
36.39 |
1.57 |
|
3 |
0.505 |
0.752 |
31.65 |
7.22 |
2.39 |
232.1 |
32.85 |
1.49 |
|
4 |
0.628 |
0.777 |
29.25 |
8.83 |
2.44 |
203.4 |
19.18 |
1.24 |
|
5 |
0.507 |
0.753 |
36.10 |
5.71 |
2.46 |
195.4 |
32.67 |
1.49 |
|
6 |
0.609 |
0.793 |
32.05 |
7.03 |
2.53 |
193.2 |
23.2 |
1.3 |
|
7 |
0.427 |
0.758 |
40.20 |
6.26 |
2.75 |
199.5 |
43.67 |
1.78 |
|
8 |
0.623 |
0.732 |
27.58 |
9.71 |
2.62 |
203.9 |
14.89 |
1.17 |
|
9 |
0.599 |
0.774 |
33.24 |
7.25 |
2.25 |
214.2 |
22.61 |
1.29 |
|
10 |
0.592 |
0.785 |
33.26 |
7.33 |
2.19 |
203.7 |
24.59 |
1.33 |
|
11 |
0.588 |
0.778 |
33.26 |
7.38 |
2.23 |
198.2 |
24.42 |
1.32 |
Table 3 Outcomes of the experimental design with the standard deviation (n = 5) in brackets
Db, Bulk Density; Dt, Tap Density; Ar, Repose Angle; Vf, Flow Rate; Hr, Residual Humidity; PS, Particle Size; CI, compressibility Index; HI, Hausner Index
|
Response |
Constant |
CSD (β1) |
LM |
LMxCSD |
R2 |
Q2 |
|
Db |
0.554* |
0.048* |
0.025* |
0.035* |
0.7383 |
0.5645 |
|
Dt |
0.769* |
-0.007* |
-0.004 |
0.014* |
0.4436 |
0.1552 |
|
Ar |
33.87* |
-3.802* |
-0.943* |
0.145* |
0.9633 |
0.8455 |
|
Vf |
7.271* |
0.871* |
0.349* |
0.321* |
0.7426 |
0.6352 |
|
Hr |
2.402* |
0.020 |
0.001 |
-0.010 |
0.0141 |
0.0000 |
|
PS |
209.11* |
-0.867 |
-0.969 |
3.615 |
0.4303 |
0.1096 |
|
CI |
27.910* |
-6.346* |
-2.901* |
-3.288 |
0.7850 |
0.6869 |
|
HI |
1.405* |
-0.129* |
-0.057* |
-0.062* |
0.7803 |
0.6399 |
Table 4 Quadratic model adjusted for each response in the tamarind dry extract.
*Statistical significant (p< 0.05)
LM, Lactose Monohydrate; CSD, Colloidal Silicon Dioxide; Db, Bulk Density; Dt, Tap Density; Ar, Repose Angle; Vf, Flow Rate; Hr, Residual Humidity; PS, Particle Size; CI, compressibility Index; HI, Hausner Index; R2, Goodness of fit; Q2, Goodness Of Prediction
Densities
Figure 1 shows effects of the amounts of LM and CSD on bulk density (A) and tapped density (B). The factor (adjuvant) showing more influence over Db was CSD, followed by CSD-LM interaction. Db significantly increased as the amount of CSD increased in TDE, from the center to the upper level. A quadratic model with statistical significance was obtained for Db. This model can explain 73.83% of the variability of Db (Table 4). Figure 1 Effects of the amounts of lactose monohydrate and colloidal silicon dioxide on bulk density (A) and tapped density (B). The LM-CSD interaction had a significant effect o the variability of Dt (Table 4). The observed behavior for Dt (Figure 1B) showed a maximum value in the region of 0.65 g of CSD which is the central level of addition of this adjuvant. In the upper and lower levels, smaller values of Dt were observed. The lowest value was observed in the upper level of CSD (1g). The effect of LM over Dt was lower than the effect of CSD. It was observed that the CI and the HI were adjusted to quadratic models with good values of R2 and Q2 that showed excellent agreement between them (differences minor than 0.15 in both cases, Table 4). There was not interaction between factors for CI (p>0.05, Table 4). Conversely, the interaction was significant for HI (p<0.05, Table 4). The CSD was the adjuvant that most improved these properties.

Figure 1 Effects of the amounts of lactose monohydrate and colloidal silicon dioxide on bulk density (A) and tapped density (B).
Flow rate and repose angle: The run number 8 showed the lowest value of Ar and the maximum value of Vf. It was noted that higher values of Vf are achieved when the amount of CSD and LM were at the higher level of addition (run 8 and 4, in that order, Table 3). The effect of the amount of CSD over Vf is approximately two times higher than the effect of LM (Table 3, figure 2A). The flow rate showed slight variation associated with the amount of LM in the dry extract. However, a great slope related increasing of CSD amount on the flow rate of the dry extract was observed. This fact shows that an increasing of the amount of CSD on the TDE improves the flow ability (Figure 2A). Figure 2 Effect of the factors (lactose monohydrate and colloidal silicon dioxide) in the flow rate (A) and repose angle (B) of the tamarind dry extract. The CSD-LM interaction showed a significant effect over Vf (p < 0.05). The adjusted model for Vf showed an R2 value of 74.26%, with a suitable agreement with Q2, which was 63.52% (Table 3). The excipient that most improved the angle of repose was CSD (Figure 2B), followed by LM. The lowest values were observed in runs 8 and 4, respectively (Table 3). The effect of both factors and the interaction between them were statistically significant (p<0.05, Table 4). The Ar was adjusted to a quadratic model (Table 4) with the best values of the goodness of fit (R2) and the goodness of prediction (Q2).
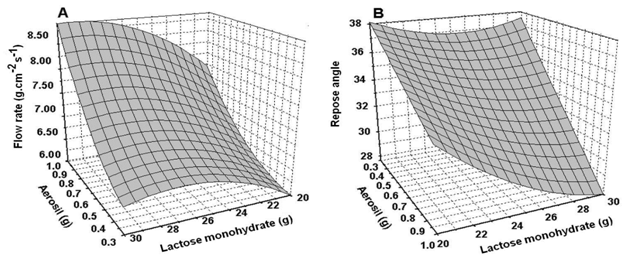
Figure 2 Effect of the factors lactose monohydrate and colloidal silicon dioxide on the flow rate (A) and repose angle (B) of the tamarind dry extract.
Residual humidity, particle size, and shape: The residual humidity and particle size did not fit any model, being the average value the best predictor of both responses (Table 4). Appropriate values (under 2.75) for Hr were obtained in all run (Table 3). Figure 3 shows a photomicrograph of the particles of the selected tamarind dry extract.
Selecting the best proportion of adjuvants : The excipients proportion used in run 8 (CSD 1.14 g and LM, 25.0 g), allowed achieving of a dry extract having good physicomechanical properties.
Phenolic and flavonoids content
The dried extracts from tamarind contain a 32.56 ± 2.28 percent of phenolic compounds as pyrogallic acid and 4.26 ± 0.87 percent of flavonoids expressed as quercetin. Both analytical methods were linear, accuracies and precise in the range of used concentrations.
Acute oral toxicity
Animals treated with the dry extract showed no signs associated with the toxic effect. The animal behavior was normal for the species. No changes in skin or pelage were observed. Mucous membranes and eyes showed appearance and normal color. The average water intake in the experimental group was 225.8 ± 5.23 ml / day and the food was 134.8 ± 8.54 g / day. In both cases, they were not statistically different from the control group (227.29 ± 4.19 ml / day for water and 140.2 ± 9.33 g / day for food). T-test = 2.48 and p-value = 0.0898 (water) and t = 1.95 and p-value = 0.0678 (for food).
The mean body weight of the animals in the control group (173.45 ± 8.94) throughout the test period was not statistically different from the average weight of the experimental group (172.74 ± 8.35) with F= 0.3000 and p = 0.8632. Levene's test (0.0016; p = 0.9683) showed that there are no statistically significant differences in the standard deviations of the body weight of the animals in both groups throughout the test period. Macroscopic analysis of liver, adrenal, kidneys, heart, spleen and lung of rat treated with TDE at the used doses in this study showed no changes in their color and morphology. Animal death was not observed at the limit dose of 2000 mg/kg.
The compressibility of powders and granulates is the result of several factors, including particle size, shape, and distribution. This property is very difficult to predict by using a mathematical model. The bulk density (Db) and the tapped density (Dt) both are significant characteristics of the powder flowability and compactability19. The model obtained for Dt presented a poor goodness of fit (R2) and a worse goodness of prediction (Q2) (Table 4). A significant interaction between CSD-LM was observed. This interaction improves the flow properties and compressibility, especially when the amounts of both increases together in the dry extract. Although Dt was not adjusted to any mathematical model, it was interesting the fact that HI and CI, which are the result of mathematical combinations of Db and Dt, were fitted to quadratic models (Table 4) with an excellent relationship between R2 and Q2 minor than 0.312.
The flowability of the powders is a very important parameter in the processing of solid pharmaceutical products.20 The flow rate and the angle of repose are two parameters associated to the flow ability of the powders and granulate. These properties describe the ability of the powder and granules to freely flow through the hoppers. Powders and granulates have good flowability when they have Ar minor than 30° and Vf greater than 7g/cm2.s.21,22 Good values of Vf and Ar were achieved, as the result of the positive effect of the increase of the amounts of adjuvants. This fact was already explained above.
The particle size and shape have great influence on the powder´s flow ability.20,23 The shape of the particles of the selected dry extract (CSD, 1.14 g, and LM, 25.0 g), has certain sphericity which results in less friction and thus increases the flow rate while the angle of repose tended to decrease. These facts lead to a better flowability of materials.15,20 The observed values of residual humidity have a positive influence on the physicomechanical properties of solids. The amount of CSD contained in the dry extract can retain moisture, keeping the grain dry, so that the flow properties were not affected. Moreover, the particles tend to fit together, reducing the friction and improved the compactability. A normal distribution of the particle size also favored the good flow and compressibility of the dry extract (data not reported). In these conditions, the new dry extract is ready to be used or included in the preparation of new solid pharmaceutical forms.13,22
The use of chemical markers is a common practice for the quality control and the dosage of natural pharmaceuticals. The selected extract showed a 32.56 ± 1.28 percent of total phenolic as pyrogallic acid and 4.26 ± 0.67 percent of flavonoids expressed as quercetin. These compounds have been reported as the responsible for antioxidant activity.24,25 and hepatoprotective.26 of the extracts of this plant. Both analytical methodologies shown to be linear, accurate and precise in the range of concentration used (Data not reported). These technics can be established for the quality control of this extract in the routine work. If it compares the obtaining method proposed here with other methods, as spray drying and freeze drying can be concluded that it was obtained a good product, in a relatively short space of time, which can be prepared in a simple way saving time and energy. In these conditions, the new dry extract is ready to use as it or can be used for the preparation of new solid pharmaceutical forms.
The plant extracts are highly sensitive to changes in temperature, humidity, and light. Thus, is very important for technologist ensure that the extract, after processing, retains its pharmacological and toxicological properties. Moreover, the evaluation of toxicity is necessary to establish the dosage regimen.27 Generally, toxic substances produce changes in the anatomy and physiology of the animals, which modify the overall clinical picture of the organisms. These changes depend on the affected organ and the duration of action. Also, depend on the total amount of absorbed substance and the age and health of the animals.18,27 The normal behavior of the animals in both groups during the study, as well as the fact that no signs of toxicity were observed during the experimental time, suggest the safety of this extract.
Body weight is perhaps the most sensitive parameter when a substance has a toxic effect on an individual.28-30 The absence of statistical differences in the consumption of water and food, allowed normal development evidenced in no statistical difference between the average weights of both groups of animals. This is an indicator of the safety of extract in this animal model. Finally, a survival rate of 100 percent and normalcy in the structure of organ tissues evaluated, evidence that subsequent processing of the liquid extract to obtaining dry extract does not cause an alteration in the active substances contained therein, that can be considered toxic. The dry extract obtained in this way was framed as a product "not qualified", within the range of toxicity classes.18
In this study, dry extracts from the leaves of Tamarindus indica L with different composition were evaluated. The experimental design allowed exploring the response surface of physicomechanical properties of the resulting dry extracts in order to select the best proportion of adjuvants to be used. The selected dried extract constituted by CSD 1.14 g and LM 25g was framed as a non-toxic substance (Not classified) according to the OECD´s toxicity class. This extract has great potential as a final product or as an active principle for capsules and/or tablets preparation. This result supports scientifically the innocuousness of the use of the leaves of this plant by the Cuban population.
This work was done with the approval and financing of the Universidad de Oriente and MediCuba Switzerland. The authors also want to thanks to CAPES and CNPq (Grand process No 402332/2013-0).
The author declares that there is not any conflict of interest.
None.

©2017 Amado, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.