International Journal of
eISSN: 2381-1803


Research Article Volume 3 Issue 4
Department of Zoology, Mizoram University, India
Correspondence: Ganesh Chandra Jagetia, Professor Department of Zoology, Mizoram University, Tanhril, Aizawl-796 004, Mizoram, India, Tel 011-389-2330724
Received: October 10, 2015 | Published: April 19, 2016
Citation: Jagetia GC, Venkatesha VA (2016) Determination of Antineoplastic Activity of Rohituka, Aphanamixis Polystachya (Wall) RN Parker in Hela Cells: Correlation with Clonogenicity and DNA Damage. Int J Complement Alt Med 3(4): 00083. DOI: 10.15406/ijcam.2016.03.00083
The antineoplastic activity of chloroform stem bark extract of rohituka, Aphanamixis polystachya (APE) used traditionally to treat spleen and liver tumors was evaluated in cultured HeLa cells by clonogenic and micronucleus assays. Treatment of HeLa cells with 0, 5, 10, 25, 50, 75 or 100µg/ml APE for different times caused a concentration-dependent reduction in the cell survival up to 6 h post-treatment (p<0.005) followed by a non-significant decline in the cells treated with APE for 24h. Therefore, 6h treatment time was considered as an optimum time for APE exposure and further studies were carried out using this treatment time. Exposure of HeLa cells to different concentrations of APE resulted in a concentration-dependent decline in the cell viability and 100µg/ml APE resulted in 72% cell death when compared with the non-drug treated control group. These results were further confirmed by clonogenic assay where, APE treatment caused a concentration-dependent decline in the clonogenicity of HeLa cells, and the surviving fraction of cells was reduced to 0.22 after treatment with 100µg/ml APE, the highest concentration screened. The inhibitory concentration (IC50) of APE was found to be 25µg/ml. The APE-induced DNA damage was estimated by micronucleus assay, where the treatment of HeLa cells with various concentrations of APE resulted in a concentration-dependent rise in the frequency of micronuclei in binucleate HeLa cells (MNBNCs) at 20, 30, and 40h post-treatment. This increase in MNBNCs was significantly higher than the baseline frequency of MNBNCs. APE treatment also increased the frequency of HeLa cells bearing more than one micronucleus (MN) indicating higher degree of DNA damage. The frequency of MNBNC increased with scoring time and a maximum elevation in the MNBNC frequency was observed at 30 h post-treatment. The MNBNC induction and clonogenicity of HeLa cells was inversely related indicating that increased MNBNC formation resulted in a corresponding reduction in the cell survival. The cell proliferation indices (PI) declined with increasing concentration of APE that increased with screening time. Our study demonstrates that APE is able to kill HeLa cells effectively and this cell killing effect of APE may be due its ability to induce DNA damage.
Keywords: hela cells, aphanamixis polystachya, cell survival, micronuclei, proliferation
APE, aphanamixis polystachyaextract; MN, micronuclei; SF, surviving fraction; MEM, minimum essential medium; DOX, doxorubicin; HeLa S3, human cervical carcinoma
Though many of the presently used anticancer drugs are originated or derived from plants, an attempt to the isolation and clinical usage of single compound has caused severe adverse side effects like, myelosuppression, alopecia, nausea, gastrointestinal, reproductive and nephrotoxicity. In addition, most of the modern chemotherapeutic agents have been reported to induce secondary malignancies due to their carcinogenic potential in patients receiving therapy.1 This necessitates a continued search for newer novel natural products and/or their semisynthetic analogs that may be useful as potential cancer chemotherapeutic drugs in the hope that it will be possible to encounter an agent/s that will be highly effective in treating neoplastic disorders without any side effect or minimum side effects when compared to the synthetic drug/s.2
Production of DNA damage is the basis of cancer treatments such as chemo- and radiotherapy. These treatments induce mitotic catastrophe, a form of cell death resulting from abnormal mitosis leading to the formation of interphase cells with multiple micronuclei. Since majority of single compounds have cytotoxic and/or DNA damaging action, one of the preliminary investigation of medicinal plant screening is to examine the effect of fractionated components on the cell proliferation as well as on the DNA of cultured neoplastic cell lines. Micronucleus assay gives indirect indication of DNA damage in the cells exposed to different cytotoxic or DNA damaging agents, since micronuclei (MN) arise from acentric fragments or whole chromosomes that lag behind during cell division.3,4 Formation of micronuclei indicates spontaneous elimination or loss of amplified sequences by tumor cells.5 Exposure of SCCVII murine carcinoma cells to chemotherapeutic drugs including cisplatin, carboplatin, etoposide, vincristine or 5-fluorouracil elevated the frequency of micronucleated binucleate cells.6 Cisplatin has also been reported to induce micronuclei in 9L tumor cells.7 Similarly, adriamycin, acyclovir or methanolic extract of Tinospora cordifolia were reported to induce micronuclei in HeLa and V79; cells.8‒11 The use of natural products, especially plants may be beneficial in cancer treatment as more than 80% of the world population still depend on plants and natural products for healthcare.12,13 Moreover, the biologic origin of plants also make them more attractive as medicines for use in humans as this will have less toxic implications and acceptability in patients will also not pose any problem.14,15
Aphanamixis polystachya Wall Parker (Amoora rohituka (Roxb.) Wight & Arn) or rohituka is distributed throughout India in evergreen forests and is a member of the family Meliaceae. The stem bark and seeds of this plant have been reported to be useful in splenomegaly, liver disorders, and treatment of tumors.16 The stem bark has been found to be useful in certain tumors including liver and spleen.17,18 The alcoholic extract of the stem bark has been reported to show anticancer activity against Friend’s leukemia and Ehrlich ascites carcinoma in mice.15,19,20 The ethyl acetate fraction of rohituka has been found to protect against the radiation-induced chromosome damage.21 Certain alkaloids like, amoorastatin and 12-hydroxyamoorastatin, present in the stem bark extract of rohituka have been reported to exhibit cytotoxic and growth inhibitory activities in murine P388 lymphocytic leukaemia cells.22 Rohituka has been reported to contain certain alkaloids and limonoids.23,24 Limonoids have been reported to possess anticancer activity in various human cancer cell lines.25 Most of the limonoids are structurally highly complex and they would never have emerged from a synthetic program alone or from a combinatorial approach to new drug discovery.2,26 Therefore it is essential to screen newer natural products for the presence of antineoplastic activity. Presence of several medicinal properties in Aphanamixis polystachya and its common usage in the Ayurvedic medicine stimulated us to screen the antineoplastic activity of the chloroform fraction of the stem bark of Aphanamixis polystachya in the cultured HeLa cells in vitro.
Chemicals
Eagle’s minimum essential medium (MEM), fetal bovine serum, L-glutamine and cytochalasin-B were purchased from Sigma Chemical Co., (St. Louis, MO, USA). Doxorubicin (DOX) was obtained as a gift from Dabur Pharmaceuticals-Oncology division, New Delhi, India, whereas all other routine chemicals were procured from Ranbaxy Fine Chemicals, Mumbai, India.
Cell line and culture
HeLa S3 (human cervical carcinoma) cells, having a doubling time of 20±2 h, procured from National Centre for Cell Science, Pune, India, was used throughout the study. The cells were routinely grown in 25 cm2 culture flasks (Techno Plastic Products, Trasadingën, Switzerland) with loosened caps containing Eagle’s minimum essential medium (MEM) supplemented with 10% fetal bovine serum, 1% L-glutamine and 50µg/ml gentamicin sulfate at 37 °C in an atmosphere of 5% CO2 in humidified air in a CO2 incubator (NuAir, Plymouth, USA).
Preparation of the extract
Aphanamixis polystachya (Wall) RN Parker or Amoora rohituka (Roxb) Wight & Arn (family-Meliaceae) or rohituka was identified by Dr. GK Bhat (a well known taxonomist) Department of Botany, Poorna Pragna College, Udupi, India and the herbarium specimen (RB-AP01) is stored with us. The bark of the tree was carefully peeled off, shade-dried, and coarsely powdered in a ball mill. The powdered bark was successively extracted with petroleum ether and chloroform in a Soxhlet apparatus for thirty cycles. The chloroform was allowed to evaporate at room temperature and the viscous extract was freeze-dried so as to obtain a fine powder of the extract. Henceforth, the chloroform stem extract of Aphanamixis polystachya will be called as APE.
Preparation of APE solution
APE was dissolved in DMSO immediately before use at a concentration of 25mg/ml and diluted with MEM as required.
Experimental
A fixed number (5 x 105) of exponentially growing HeLa cells were seeded into several culture flasks (Techno Plastic Products, Trasadingën, Switzerland) and were allowed to attach for 48h. The cell cultures were divided into the following groups:
MEM group: The cells of this group were treated with 4µl/ml DMSO
APE gorup: This group of cells was treated with 0, 5, 10, 25, 50, 75 or 100µg/ml APE for 1, 2, 4, 6, 8, 12, 16 or 24 h.
DOX group: The cells of this group were exposed to 0, 2, 4, 6, 8, 10 or 12µg/ml doxorubicin (positive control).
The following studies were carried out to ascertain the antineoplastic activity of rohituka in cultured Hela cells.
Evaluation of optimum treatment time
The optimum treatment duration for APE in HeLa cells was determined by estimating cell viability.26 The results obtained by this assay were further confirmed by clonogenic assay.27
Pratt and Willis test
A separate experiment was conducted to determine the optimum APE exposure time, where grouping and other conditions were similar to that described in the experimental section. Briefly, 5 x 10M4 log phase cells were seeded into 35cm2 culture dishes (Cellstar, Greiner, Germany) in triplicate for each drug concentration. 24h after initiation of the cultures the cells were treated with 0, 5, 10, 25, 50, 75 or 100µg/ml APE or 0, 2, 4, 6, 8, 10 or 12µg/ml doxorubicin for 1, 2, 4, 6, 8, 12, 16 or 24h. After the elapse of stipulated time, the drug-containing medium was replaced with the fresh drug-free medium and the cells were left undisturbed in a CO2 incubator at 37°C. The whole experiment was terminated after 72h of initiation and the cells were dislodged by trypsin-EDTA treatment. The viability of cells was determined by trypan blue dye exclusion test using a hemocytometer (AO, American Optical Co., Cambridge, USA) under an inverted microscope (Ernst Leitz, Wetzlar GmbH, Wetzlar, Germany).
Clonogenic assay
The clonogenic potential of HeLa cells after APE treatment was evaluated by the method of Puck & Marcus27 by conducting a separate experiment. The optimum APE treatment duration was determined by dividing the cells into two groups as described in the experimental section except, that 200-300 exponentially growing cells were plated on to several individual culture dishes (Cellstar, Greiner, Germany) containing 5ml drug free medium in triplicate for each drug concentration for each group. The cells were allowed to grow for 24h and the cells of all groups were treated with various concentrations of APE or doxorubicin for 1, 2, 4, 6, 8, 12, 16 or 24h as indicated in the earlier section. The drug-containing media was replaced with APE free medium after the elapse of stipulated time. The cells were incubated and the experiment was terminated on 11th day. The cultures from various groups were stained with 1% crystal violet in 1% methanol. The colonies of cells were scored using stereozoom microscope (E Wild M3, Wild Heerbrugg Ltd., Heerbrugg, Switzerland). The clones containing a minimum of 50 or more cells were scored as a colony. The plating efficiency was determined and surviving fraction (SF) calculated.
The data were fitted on to linear quadratic equation:
The optimum treatment time for APE exposure was determined on the basis of cell viability and surviving fraction. Treatment of HeLa cells with various concentrations of APE showed a maximum cell kill for 6h APE treatment. Therefore, further experiments were carried out using this APE treatment time.
Effect of various concentrations of APE on cell survival and micronuclei formation
The experimental design has been essentially similar to that described above except that HeLa cells were exposed to DMSO or APE for 6h, thereafter, the APE-containing medium was removed, and the cells were dislodged from the culture flasks by trypsin-EDTA treatment. The cells were divided into two parts and the following studies were carried out:
Clonogenic assay
One part of the cells was used for clonogenic assay, where 200 cells from each concentration of APE were seeded in quadruplicate. The details of the assay have been described earlier.
Micronucleus assay
The other part of cells left after clonogenic assay was used for micronucleus study, where 3 x 105 cells were inoculated in triplicate for each drug concentration. The micronuclei were prepared according to the modified method of Fenech & Morley.28 Briefly, the cells were allowed to attach for 6h; after which the cells were treated with 3µg/ml of cytochalasin-B to inhibit cytokinesis. The cells were left undisturbed and were allowed to grow for another 14, 24 or 34h, depending on the assay time. The cell cultures were terminated at 20, 30 and 40 h post-drug-treatment. The medium containing cytochalasin-B was removed; the cells were washed with PBS, dislodged by trypsin EDTA treatment and centrifuged. The cell pellet was disturbed and subjected to mild hypotonic treatment (0.75% ammonium oxalate) at 37°C, centrifuged again and the resultant cell pellet was fixed in Carnoy’s fixative 3:1 (methanol:acetic acid). After centrifugation, the cells were resuspended in a small volume of fixative and spread on to precleaned coded slides to avoid observer’s bias.
The slides containing cells were stained with 0.125% acridine orange (BDH, England, Gurr Cat. no. 34001 9704640E) in Sorensen’s buffer (pH 6.8) and subsequently washed twice in the Sorensen’s buffer. The slides mounted in Sorensen’s buffer were observed under a fluorescent microscope equipped with 450-490nm BP filter set with excitation at 453nm (Carl Zeiss, Photomicroscope III, Oberkochen, Germany) using a 40X Neofluar objective. A minimum of one thousand binucleate cells with well-preserved cytoplasm was scored for each concentration and the frequency of micronucleated binucleate cells (MNBNC) was determined. The micronucleated cells were scored according to the criteria of Kirsch-Volders et al. 29,30 and Fenech et al. 31. The data regarding the cell proliferation kinetics were also collected, where the frequencies of mono-, bi-, tri- and tetranucleate cells were determined. The proliferation indices (PI) were calculated from the mean of 4000 cells for each drug concentration.
The PI was calculated as follows:
PI = (1 x N) + (2 x 2N) + (3 x 3N) + (4 x 4N) ÷1000
Where: N = total mononucleate cells, 2N = total binucleate cells, 3N = total trinucleate cells, and 4N = total tetranucleate cells.
The regression analyses were carried out using for linear and for linear quadratic equations.
Statistical analyses
The statistical significance between the treatments was analysed using one-way ANOVA for clonogenic, and micronucleus assays as well as for the proliferative indices. The data were confirmed by repetition of the experiments. The test of homogeneity has been applied and data of two experiments did not differ significantly from one another. Hence the data of both the experiments are combined and presented as means. Appropriate post-hoc tests were used for multiple comparisons. Solo 4 (BMDP Statistical Software Inc., Los Angeles, CA) was used for statistical analyses.
The results are expressed as percent viability, surviving fraction (SF), micronuclei frequency per thousand cells, and cell proliferation indices as mean ± SEM (standard error of the mean) in Table 1-3 & Figure 1-5.

Figure 1 Effect of different treatment times on the percent viability of HeLa cells treated with different concentrations of chloroform extract of Aphanamixis polystachya (a) or doxorubicin (b) (used as positive control) as determined by Pratt and Willis test.

Figure 2 Effect of different treatment times on the survival of HeLa cells treated with different concentrations of chloroform extract of Aphanamixis polystachya (a) or doxorubicin (b) (used as positive control).
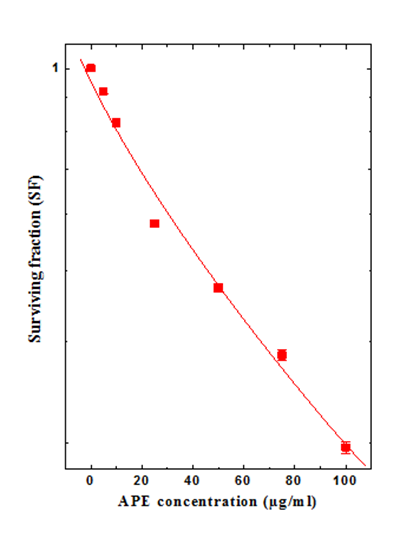
Figure 3 Effect of different concentration of chloroform extract of Aphanamixis polystachya on the survival of HeLa cells.

Figure 4 Effect of various concentrations of chloroform extract of Aphanamixis polystachya on the micronuclei formation in HeLa cells at different post-treatment times: a: one MN; b: two MN; c: multiple MN and d; total MN Closed squares, 20 h; Closed circles, 30 h; Closed triangles, 40 h post-treatment.
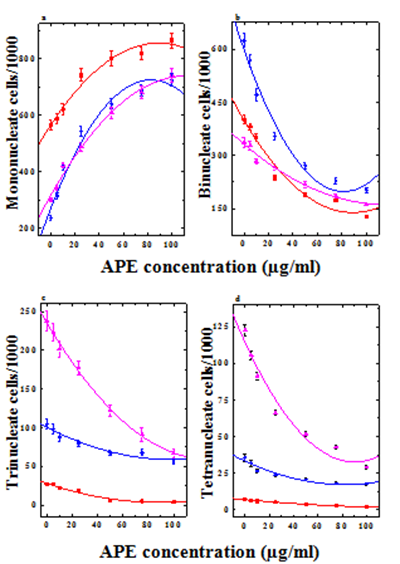
Figure 5 Effect of different concentrations of CAP on the HeLa cell proliferation kinetics at various post-treatment times. a: mononucleate cells; b: binucleate cells;c: trinucleate cells and d: tetranucleate cells. Closed squares, 20 h; Closed circles, 30 h; Closed triangles, 40 h post-treatment.
Evaluation of optimum treatment time for APE treatment
Pratt and Willis test: A maximum number of surviving cells (>99%) were observed in non-drug-treated control group. Treatment of HeLa cells with various concentrations of APE for 0, 1, 2, 4, 6, 8, 12, 16 or 24h caused a concentration dependent decline in the number of surviving cells. Similarly, viability of cells declined with the length of treatment time and a maximum reduction in cell viability was observed for 6 h treatment (Figure 1). Thereafter, the difference between 6 h (p<0.005) and other longer treatment times was statistically non-significant (Table 1). However, the effect of treatment time on cell viability was significantly different up to 4h in comparison with 6h APE treatment (Table 1). The inhibitory concentration (IC50) of APE was found to be 25µg/ml for 6h exposure time. Exposure of doxorubicin to HeLa cells also inhibited the viability of cells in a concentration-dependent fashion (Figure 1) akin to APE treatment, except that the highest effect was observed for 10µg/ml of doxorubicin after 4h drug exposure (Table 1).
Concentration (µg/ml) |
Cell Viability (%) ± SEM |
|||||||||
Drug-Treatment Time (h) |
||||||||||
0 |
1 |
2 |
4 |
6 |
8 |
12 |
16 |
24 |
||
APE |
0 |
99.32±0.14 |
99.94±0.15 |
99.86±0.14 |
99.27±0.16 |
99.69±0.16 |
98.44±0.16 |
98.97±0.23 |
99.35±0.28 |
99.24±0.31 |
5 |
99.57±0.13 |
96.85±0.13 |
93.42±0.12 |
80.74±0.13 |
73.54±0.14 |
72.18±0.15 |
72.16±0.17 |
71.30±0.14 |
70.42±0.16 |
|
10 |
99.61±0.15 |
91.00±0.13 |
83.17±0.13 |
73.79±0.13a |
66.32±0.11 |
65.91±0.12b |
65.58±0.12b |
65.04±0.12b |
64.97±0.14b |
|
25 |
98.95±0.14 |
86.89±0.13 |
75.43±0.1b |
66.61±0.1c |
52.76±0.05c |
51.49±0.05c |
51.33±0.05c |
50.02±0.05c |
49.61±0.06c |
|
50 |
99.26±0.15 |
77.01±0.12 |
65.09±0.1b |
58.53±0.10c |
41.01±0.05c |
40.66±0.04c |
40.39±0.04c |
39.75±0.04c |
39.19±0.04c |
|
75 |
99.48±0.21 |
64.72±0.12 |
55.01±0.1b |
47.76±0.10c |
35.05±0.04c |
34.95±0.03c |
34.82±0.03c |
34.52±0.0 |
34.11±0.04c |
|
100 |
99.39±0.29 |
59.80±0.12 |
48.83±0.1b |
39.70±0.09c |
28.19±0.03c |
27.75±0.03c |
27.52±0.03c |
26.89±0.03c |
26.20±0.04c |
|
DOX |
2 |
99.42±0.32 |
96.41±0.14 |
89.74±0.13 |
82.62±0.13 |
82.53±0.13 |
82.23±0.16 |
82.20±0.18 |
81.58±0.13 |
80.71±0.15 |
4 |
99.49±0.15 |
92.52±0.13 |
85.25±0.14 |
77.34±0.12a |
77.12±0.12a |
77.10±0.13a |
77.45±0.12b |
76.85±0.11b |
76.15±0.12c |
|
6 |
99.35±0.14 |
89.29±0.14 |
84.79±0.12 |
70.00±0.11c |
70.35±0.12c |
70.18±0.13c |
69.48±0.13c |
68.54±0.10c |
67.65±0.13c |
|
8 |
99.24±0.16 |
85.16±0.14 |
79.18±0.12 |
59.66±0.08c |
58.06±0.09c |
57.44±0.90c |
56.82±0.08c |
56.34±0.08c |
55.11±0.11c |
|
10 |
99.16±0.15 |
82.29±0.14 |
71.79±0.12 |
52.00±0.11c |
51.65±0.12c |
51.38±0.13c |
51.40±0.13c |
50.74±0.10c |
50.05±0.13c |
|
12 |
99.08±0.14 |
63.28±0.12 |
49.68±0.11 |
24.69±0.08c |
23.23±0.08c |
22.79±0.08c |
21.97±0.07c |
20.94±0.08c |
19.66±0.10c |
|
Table 1 Effect of different treatment times on the viability of HeLa cells treated with various doses of Aphanamixis polystachya
APE = chloroform fraction of the stem bark of Aphanamixis polystachya ; DOX=doxorubicin, a positive control; and SEM = standard error of the mean.
p < a = 0.05, b = 0.01, c = 0.005, no symbols = not significant when compared with the concurrent non-drug treated group.
5 x 104 log phase HeLa cells were seeded in several culture flasks. On the following day the cells were treated with various doses of CAP for different times as indicated above. After the treatment time the drug-containing medium was replaced with fresh medium and the cultures were re-incubated further up to 72h when they were harvested, stained with trypan blue and counted. The results are the mean of three independent trials/drug concentration/treatment time and expressed as % viability
Binucleate Cells Bearing Micronuclei |
APE (µg/ml) |
Frequency of Micronucleated Binucleate Cells per 1000 ± SEM |
||
Post-Treatment Scoring Time (h) |
||||
20 |
30 |
40 |
||
One MN |
0 |
5.78±0.63 |
16.50±1.66 |
7.33±0.80 |
5 |
8.14±0.83 |
20.84±1.10 |
12.64±0.96a |
|
10 |
11.61±1.08a |
31.25±2.24a |
23.50±1.26b |
|
25 |
25.7±2.04b |
74.67±5.18b |
46.67±3.47b |
|
50 |
34.81±2.51b |
91.13±6.20b |
58.50±3.90b |
|
75 |
42.33±3.16b |
99.54±6.11b |
67.86±4.47c |
|
100 |
47.48±3.88b |
107.60±7.32b |
75.78±5.65c |
|
r2 |
0.99 |
0.97 |
0.98 |
|
Two MN |
0 |
0.21±0.03 |
0.54±0.07 |
0.42±0.03 |
5 |
1.17±0.09b |
2.40±0.16b |
1.73±0.11b |
|
10 |
2.48±0.14c |
4.64±0.32b |
2.96±0.20b |
|
25 |
5.16±0.37c |
8.02±0.52b |
6.12±0.48b |
|
50 |
6.31±0.38c |
9.73±0.73b |
7.78±0.56b |
|
75 |
6.90±0.48c |
10.80±0.80b |
8.84±0.65b |
|
100 |
7.86±0.50c |
12.92±0.94b |
9.96±0.76b |
|
r2 |
0.96 |
0.95 |
0.97 |
|
Multiple MN |
0 |
0.30±0.03 |
1.12±0.10 |
0.48±0.03 |
5 |
0.83±0.05b |
1.60±0.08 |
1.07±0.06b |
|
10 |
1.26±0.08b |
2.06±0.14a |
1.61±0.09b |
|
25 |
2.20±0.12c |
3.48±0.23b |
2.56±0.14b |
|
50 |
2.50±0.13c |
4.10±0.31b |
3.06±0.21b |
|
75 |
3.06±0.17c |
4.71±0.35b |
3.61±0.36b |
|
100 |
3.38±0.23c |
5.43±0.43b |
4.12±0.37b |
|
r2 |
0.96 |
0.97 |
0.97 |
|
Total MN |
0 |
6.29±0.63 |
18.16±1.36 |
8.23±0.96 |
5 |
10.14±0.86 |
24.84±1.84 |
15.44±1.22a |
|
10 |
15.35±1.21a |
37.95±2.31a |
28.07±2.40a |
|
25 |
33.06±2.16b |
86.17±3.62c |
55.35±3.75b |
|
50 |
43.62±2.42c |
104.96±4.77c |
69.34±4.30b |
|
75 |
52.29±2.71c |
115.05±5.33c |
80.31±4.27c |
|
100 |
58.72±3.10c |
125.95±5.19c |
89.86±4.66c |
|
r2 |
0.99 |
0.97 |
0.98 |
|
Table 2 Micronuclei induction in HeLa cells by various concentrations of chloroform extract of Aphanamixis polystachya at different post-treatment times
p < a = 0.05, b = 0.01, c = 0.005, no symbols = not significant when compared to non-drug treated control.
APE = Chloroform extract of the stem bark of Aphanamixis polystachya; SEM = Standard error of the mean;
MN = micronuclei; r2 = coefficient of correlation
Clonogenic assay: Treatment of HeLa cells with DMSO for different times did not adversely affect their clonogenicity, as evidenced by non-significant changes in the survival of HeLa cells (Figure 2). Exposure of HeLa cells to APE for 0, 1, 2, 4, 6, 8, 12, 16 or 24h resulted in a time and concentration-dependent decline in the clonogenicity of cells. A 50% reduction in the surviving fraction of treated cells was obtained after treatment with 50µg/ml APE for 1h. An identical effect was discernible in the cells treated with 25µg/ml APE for 6h (Figure 2). Surviving fraction of cells reduced significantly with exposure time up to 6h depending on the APE concentration and a highest decline in cell survival was observed for 100µg/ml. An optimum inhibitory concentration (IC50) was found to be 25µg/ml for 6h treatment when compared to the non-APE treated controls i.e. 0 concentrations (Figure 2). Since maximum cell killing effect was observed for 6h APE exposure, this treatment time was considered as an optimum treatment time and further studies were undertaken using this time for APE treatment. Treatment of HeLa cells with different doxorubicin concentrations also reduced the surviving fraction (SF) of cells in a time dependent manner and a highest reduction of cell survival was observed after 4h exposure for 10µg/ml DOX (Figure 2).
Effect of various concentrations of APE on cell survival and micronuclei formation
Clonogenic assay: Treatment of HeLa cells with various concentrations of APE resulted in a concentration- and time-dependent decline in the SF (Figure 3). The lowest concentration of APE i.e. 5µg/ml, reduced the SF of cells by 10%, whereas increasing concentration of APE resulted in a corresponding decline in the clonogenicity of HeLa cells until a nadir was reached at 100µg/ml APE, the maximum concentration evaluated for cytotoxicity (Figure 3). Treatment of HeLa cells with 25µg/ml APE resulted in a 40% reduction in the cell survival when compared with 5µg/ml APE treatment, whereas this decline in SF was approximately 50% when compared with the non-APE treated control (Figure 3). Hence this concentration was considered as IC50 concentration of APE.
Cell proliferation index
The base line proliferation index (PI) of HeLa cells was found to be 2 at all the post-treatment times studied. The frequency of mononucleate cells showed a concentration-dependent elevation (Figure 5a), which was significantly higher at all the concentrations of APE at all the post-treatment times when compared with the non-drug-treated controls (Table 3). In contrast, treatment of HeLa cells with various concentrations of APE resulted in a concentration-dependent decline in the frequency of binucleate cells at all the post-treatment times (Figure 5b). This decline in binucleate cells was significantly higher for all the APE concentrations at 20, 30 and 40h post-treatment resulting in a 50 to 90% reduction in PI (1.5 and 1.14) in the cells treated with 25 and 100µg/ml APE, respectively at 20h post-treatment (Table 3). The frequency of multinucleate (tri and tetranucleate) cells also showed a concentration-dependent decline indicating a block in the cell division. Multinucleate cells were almost absent at 20h after exposure to 25-100µg/ml APE (Figure 5c & 5d). This decline in multinucleate cells was significant at 20 and 30h post-treatment when compared with non-drug treated controls (Table 3). The PI showed a time dependent rise for all the APE concentrations and a maximum PI was recorded at 40h post-APE treatment (Figure 5).
Cell Types Scored |
APE (µg/ml) |
Frequency of Nucleated Cells Per 1000 ± SEM |
||
Post-Treatment Scoring Time (h) |
||||
20 |
30 |
40 |
||
Mononucleate |
0 |
565.57±18.68 |
237.27±8.50 |
302.58±6.60 |
5 |
588.64±19.20 |
314.5±9.68 |
343.48±8.86 |
|
10 |
621.64±20.20 |
414.5±10.30 |
424.48±10.16 |
|
25 |
741.86±22.50 |
543.81±18.10 |
439.6±14.70 |
|
50 |
801.42±25.51 |
640.62±18.20 |
609.6±19.76 |
|
75 |
819.76±21.76 |
685.68±20.26 |
680.6±21.81 |
|
100 |
865.89±26.88 |
725.69±21.52 |
742.83±24.86 |
|
r2 |
0.97 |
0.97 |
0.99 |
|
Binucleate |
0 |
401.16±12.10 |
623.36±19.48 |
336.76±13.90 |
5 |
381±9.65 |
567.8±17.35 |
327.89±12.47 |
|
10 |
351±9.31 |
470.8±16.21 |
282.89±8.10 |
|
25 |
235.54±7.15 |
353.68±10.52 |
267.52±8.38 |
|
50 |
190±5.20 |
271.6±8.20 |
217.52±7.41 |
|
75 |
173.77±4.08 |
228.65±9.41 |
186.57±4.52 |
|
100 |
128.71±4.00 |
201.97±8.60 |
162.55±3.50 |
|
r2 |
0.96 |
0.97 |
0.98 |
|
Trinucleate |
0 |
26.27±0.65 |
104.38±6.26 |
238.1±12.70 |
5 |
26.49±0.71 |
97.71±6.30 |
223.73±11.12 |
|
10 |
22.49±0.57 |
87.71±5.20 |
201.72±10.50 |
|
25 |
18.22±0.44 |
79.51±4.43 |
176.95±8.18 |
|
50 |
5.64±0.34 |
67.4±3.45 |
121.95±7.00 |
|
75 |
4.85±0.34 |
67.68±3.95 |
90.95±8.88 |
|
100 |
3.82±0.27 |
55.48±2.86 |
66.70±5.52 |
|
r2 |
0.97 |
0.94 |
0.99 |
|
Tetranucleate |
0 |
7.16±0.66 |
35.5±2.55 |
122.69±3.68 |
5 |
6.2±0.58 |
31.86±2.10 |
105.57±3.15 |
|
10 |
5.72±0.58 |
26.37±1.10 |
91.32±2.41 |
|
25 |
5.1±0.43 |
23.5±1.22 |
66.2±2.06 |
|
50 |
3.56±0.29 |
20.88±0.95 |
51.44±1.56 |
|
75 |
2.84±0.34 |
18.32±0.80 |
42.78±1.26 |
|
100 |
1.66±0.20 |
17.17±0.68 |
28.9±1.10 |
|
r2 |
0.98 |
0.92 |
0.96 |
|
Proliferative index (PI) |
0 |
1.46 |
1.92 |
2.18 |
5 |
1.44 |
1.82 |
2.09 |
|
10 |
1.4 |
1.71 |
1.96 |
|
25 |
1.28 |
1.57 |
1.82 |
|
50 |
1.2 |
1.44 |
1.61 |
|
75 |
1.18 |
1.4 |
1.49 |
|
100 |
1.14 |
1.35 |
1.38 |
|
Table 3 Alteration in the cell proliferation kinetics of HeLa exposured of different concentrations of choloroform extract of Aphanamixis polystachya for 6 h
APE = chloroform fraction of the stem bark of Aphanamixis polystachya ; SEM = standard error of the mean;
r2 = coefficient of correlation
Proliferation kinetics (PI), a measure of cell division kinetics was calculated from the mean of at least 4000 cells/drug concentration using the formula:
PI = [(1x N) + (2x 2N) + (3x 3N) + (4x 4N)] ÷ 1000
N = mononucleated cells; 2N = binucleated cells; 3N = trinucleated cells; 4N = tetranucleated cells.
Biological response
The biological response was evaluated by correlating the cell survival and micronuclei induction, where the surviving fraction was plotted on X-axis and micronuclei frequency on Y-axis. The dose response relationship was linear (r2 = 0.97, 0.98, 0.97 for 20, 30 and 40h, respectively). The cell survival declined with the increasing micronuclei frequency indicating an inverse relationship between surviving fraction and micronuclei formation (Figure 6).
Due to the identification of several anticancer drugs from various plants, random screening for compounds leading to candidates for anticancer agents is continually performed, despite recent progress in molecular and cellular research on cancer therapy.32 The plants synthesize a variety of chemicals as secondary metabolites to combat stress, fight against the predators, as nutrients and to carry out various physiological activities.33 These plant metabolites have been useful in human healthcare in various ways and many of these metabolites have provided modern anticancer drugs.32 Therefore, it is necessary to screen medicinal plants for new drug discovery and also for combinatorial chemistry. The antineoplastic activity of the stem bark extract of Aphanamixis polystachya (APE) has been evaluated in cultured HeLa cells in the present study.
HeLa cells are frequently employed as model cancer cells to determine the cytotoxic effects of chemical or physical agents.34 The cytotoxic effect of APE increased with time up to 6h post-treatment and remained almost unaltered thereafter as evident by cell viability and clonogenic assays. An identical effect was observed earlier when HeLa cells were treated with doxorubicin, acyclovir, azidothymidine or extract of Tinospora cordifolia.8,21,35,36 The cytotoxic effect of APE increased with increasing concentrations and an IC50 concentration was found to be 25µg/ml. An almost complete inhibition of cell division was discernible at a concentration of 100µg/ml APE as indicated by low PI and very low clonogenicity. The reports regarding the cytotoxic effect of APE in vitro are unavailable. However, triterpenoid amooranin present in Aphanamixis polystachya has been reported to be cytotoxic to HeLa, MCF-7 and SW620 human colon carcinoma cells in vitro.37,38 The alcoholic extract of the stem bark of Aphanamixis polystachya (AP) showed cytotoxic activity on Ehrlich ascites carcinoma cells in vivo.15 Similarly, other antineoplastic agents like, doxorubicin, vinblastine, taxol and teniposide (VM-26) have been reported to induce cytotoxicity in V79 cells.11,39‒42 Treatment of HeLa, MCF-7, HL 60, or KB cells to alcoholic extract of the stem bark of Alstonia scholaris or echitamine chloride have been reported to induce cytotoxicity earlier.8,20,43,44 The cytotoxic effect of APE is reflected in proliferation indecies (PI), where a concentration dependent reduction in PI was observed at all post-treatment times in cells treated with various concentrations of APE. Its petroleum ether extract has been reported to be cytotoxic in cultured human breast cell lines earlier.45
The chemotherapeutic agents usually act by eliciting DNA damage by inducing single or double strand breaks or other types of DNA damages that may be converted into DNA double strand breaks that prove fatal to the cells. Therefore we were interested to determine whether the APE is able to damage the cellular DNA or not and the observed cell killing effect is due to DNA damage? The micronuclei are surrogate markers of DNA damage, which are produced by acentric fragments or a whole chromosome that could not attach to the mitotic spindle during cell division and are excluded from the main nuclei or due to defective spindle fibers or kinetochores. The mechanisms involved in the formation of micronuclei may be different depending on the action of the drug. They may be produced due to chromosome breakage (clastogenesis) or spindle disruption (aneugenesis).3,4 The micronuclei can be studied with ease and do not require a great skill unlike chromosome aberrations and their study provides the information which is forthcoming from the analysis of chromosome aberrations.11
Exposure of HeLa cells to various concentrations of APE resulted in a concentration dependent elevation in the frequency of micronuclei. APE not only induced one MNBNC, but also cells bearing two and multiple MNBNC, indicating that it induced complex multiply site of DNA damage of non-reparable nature. The reports regarding the induction of micronuclei by APE are unavailable. This is probably the first report describing the APE-induced DNA damage in HeLa cells. Similarly, doxorubicin, extract of Tinospora cordifolia, acyclovir and azidothymidine have been reported to increase the frequency of micronuclei in a concentration-dependent manner in HeLa cells earlier.8‒10,35 The treatment of V79 cells with doxorubicin has been found to increase the micronuclei frequency in concentration dependent manner.11 The other chemotherapeutic drugs including vindesine, vinblastine, taxol and teniposide increased the frequency of micronuclei in V79 cells in a concentration dependent fashion.39‒42,46 The frequency of MNBNC increased with time, and a peak was observed at 30h post-treatment which may be due to the APE-induced cell cycle delay. A similar effect has been reported earlier in HeLa cells.8 The micronuclei frequency will be highest immediately after first cell division, which seems to be the reason of maximum frequency of micronuclei at 30h post treatment and decline with subsequent cell division. The decline in micronuclei at later scoring times is due to the dilution owing to cell division or cell death as observed in the present study. The results of cell proliferation indices support this contention.
Micronuclei induction and cell survival are found to be closely related. This is expected as the micronuclei are the surrogate marker of DNA damage and cells expressing DNA damage are dying cells. Once the cells express MN they lose clonogenic potential owing to the loss of significant amount of genome and eventually die. The nuclear alterations and micronuclei formation are the major lethal events resulting in the cell killing of HeLa cells.11,47 This is supported by a decline in surviving fraction with increasing MNBNC in the present study, indicating an inverse relationship between cell survival and MNBNC induction. The relationship between micronuclei induction and cell survival has been linear. A similar relationship has been reported for HeLa cells after treatment with various doses of acyclovir and azidothymidine.9 However, a linear quadratic relationship has also been reported for HeLa cells treated with doxorubicin or Tinospora cordifolia8,9 and also for V79 cells treated with various doses of taxol, vindesine or teniposide or doxorubicin.11,39‒42 In contrast, Bush & McMillan48 did not find any relationship between micronuclei induction and cell survival.
The frequency of micronuclei in L929 cells was influenced by drugs like etoposide (topoisomerase II inhibitor) and H-7, Go6983 (PKC inhibitors) known to play an important role in signaling and execution of apoptosis suggesting that micronuclei induction mediated by topoisomerase II and protein kinase pathways leads to the formation of apoptotic cells leading to cell death.49 A wide range of anticancer agents like, mitomycin C, doxorubicin, epirubicin, cisplatin, carboplatin, etoposide, vincristine, 5-fluorouracil, methotrexate, nimustine and dacarbazine have been reported to induce micronuclei in SCCVII murine carcinoma cells, where a good correlation between the formation of micronuclei and cell survival has been reported.6 Similarly, cisplatin and 5-fluorouracil have been found to increase micronuclei and reduce clonogenicity of rodent ovarian carcinoma cells.50
The exact mechanism of action of APE is not known. The increased cytotoxicity of APE in HeLa cells may not be due to a single mechanism, but may be due to the operation of multiple mechanisms in concert with one another. Depletion in clonogenicity may mainly be due to the initiation of DNA damage by APE. This contention is supported by a concentration-dependent rise in the MNBNC frequency in HeLa cells in the present study. Due to a significant loss of genome, the APE treatment may have killed HeLa cells efficiently. This DNA damage may have been caused by the induction of oxidative stress by APE that in turn would have induced free radicals thereby attacking the genome. Oxidative stress caused by biochemical dysfunction like glutathione50 depletion results in chromatin dysfunction such as single strand and double strand DNA fragmentation leading to cell death. Under depleted GSH condition, endogenously generated hydrogen peroxide might be converted into hydroxyl free radicals. These hydroxyl radicals cause lipid peroxidation and in addition result in the formation of giant DNA fragments and internucleosomal DNA fragments which ultimately drive the cell to necrosis or apoptosis, respectively.51 The APE treatment may also have stimulated apoptosis of cells thereby reducing the clonogenicity. In the present study, APE has induced apoptosis after 6h treatment where 15% of the exposed cells became apoptotic (data not shown). Amooranin, a triterpenoid present in Aphanamixis polystachya has been reported to induce DNA damage and apoptosis earlier.37 APE treatment may also arrest the cells in G2+M phase and killing them subsequently. Depletion in the proliferation index indicates that APE treatment may be doing so in reality. Amooranin has been reported to arrest cells in G2+M phase.52 Inhibition of CDK2, CDK4 and serine/threonine kinases may have also contributed to its cytotoxic activity. The flavopiridol,53 a semisynthetic derivative of rohitukine has been reported to inhibit CDK2 CDK2, CDK4 and serine/threonine kinases.54‒56 The observed antineoplastic action of APE may be due to inhibition of NF-κB, and COX-II. Amooranin has been found to suppress NF-κB, and COX-II activation.38,57 The suppression of topoisomerase II activity by APE is also not ruled out as it produced micronuclei efficiently. Since topoisomerase II plays an important role during replication. It is also speculated that APE may have down regulated the Nrf2 gene that may have increased the oxidative stress in HeLa cell leading to effective cell kill.58
The DNA damaging and cell killing effects of APE may be partially attributed to the presence of alkaloids rohitukin, amooranin, amoorastatin and 12α-hydroxyamoorastatin, which have been reported to kill neoplastic cells.23 The action of APE may not only be due to the presence of amooranin and rohitukin alkaloids but may also be due to the presence of complex limonoids like, polystachin, prieurianin, hispidin C, aphanamixin and aphananin23,24,59‒64 whose concerted action may have increased the DNA damage and greater cell kill in the present study.
APE treatment induced cytotoxic effects in HeLa cells in a concentration-dependent decline in the clonogenicity of cells with an IC50 of 25µg/ml. The cytotoxic effect of APE may be due to induction of DNA damage in the form of micronuclei and induction of apoptosis. The depletion in GSH might have induced oxidative stress and the free radicals leading to DNA damage and ultimately to cell death. APE may also have induced cell cycle blockage at G2+M phase. The inhibition of topoisomerase may have contributed to increased DNA double strand breaks and subsequently micronuclei formation by APE in HeLa cells. The APE may have also suppressed the activation of NF-κB, COX-II and Nrf2 genes, which may have contributed in various ways to increase its cell killing effect. The cytotoxic effects of APE may be due to the presence of alkaloids including alkaloids rohitukin, amooranin, amoorastatin and 12α-hydroxyamoorastatin and complex liminoids in it.
The authors are thankful to the Council of Scientific and Industrial Research, Indian Council of Medical Research, Govt. of India for extending the financial support to carry out this study.
Author declares there are no conflicts of interest..
None.

©2016 Jagetia, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.